The only pure covalent bonds occur between identical atoms. In these compounds, the negatively charged portion is a polyatomic ion (or anion), and the positively charged portion consists of cations. Covalent Is NaI ionic or molecular? Huang et al. See answer (1) Best Answer. It has a hexagon shape in the middle consisting of 5 carbons and 1 oxygen. WebThe following sections provide descriptions of the major types of crystalline solids: ionic, metallic, covalent network, and molecular. In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. This compound is made of C-H bonds, C-O bonds and O-H bonds. so sugar is compound involving pure covalent bonds. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. Would you like to make it the primary and merge this question into it? Continue reading >>, Does glucose form an ionic or covalent bond? Is dextrose ionic or covalent? 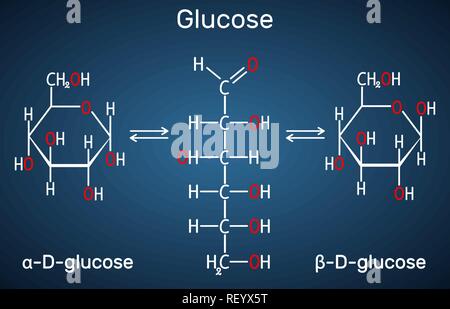 Covalent bonds are only able to occur when elements that are close together on the periodic table of elements form together and begin sharing electrons. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements. Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding.
Covalent bonds are only able to occur when elements that are close together on the periodic table of elements form together and begin sharing electrons. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements. Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding.  The number of shared electrons depends on the number of electrons needed to complete the octet. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Molecular. How do you download your XBOX 360 upgrade onto a CD? You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Just use a periodic table which includes it and read it for the selected element. Molecular (covalent) compounds are between only non-metals. What can you say about each other properties; melting point and electrical conductivityto the ionic or covalent character of the compound?4)Predict the followingoSolubility of sodium iodide in waterNaL is an iconic compound since its composed of a metal and non-metal.
The number of shared electrons depends on the number of electrons needed to complete the octet. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Molecular. How do you download your XBOX 360 upgrade onto a CD? You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Just use a periodic table which includes it and read it for the selected element. Molecular (covalent) compounds are between only non-metals. What can you say about each other properties; melting point and electrical conductivityto the ionic or covalent character of the compound?4)Predict the followingoSolubility of sodium iodide in waterNaL is an iconic compound since its composed of a metal and non-metal.  \nNow, the Oxygen needs 1 electron to complete it's outer shell and the Carbon needs 3 to complete it's outer shell. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). Why? Now, when salt is dissolved in water, somethinginteresting happens. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. Ionic bonds, on the other hand, form when one atom donates an electron to another atom.
\nNow, the Oxygen needs 1 electron to complete it's outer shell and the Carbon needs 3 to complete it's outer shell. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). Why? Now, when salt is dissolved in water, somethinginteresting happens. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. Ionic bonds, on the other hand, form when one atom donates an electron to another atom.  These types of bonds are different from a covalent sugar bond because sugar is made up of two nonmetal elements. Then, draw 1 Hydrogen connected to the Carbons, by themselves. 2.27 B). The vacancies are implied because the dots are alone and not paired. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. Already a member? When the distance is increased and the shielding is also increased, it causes a decrease in nuclear attraction.
These types of bonds are different from a covalent sugar bond because sugar is made up of two nonmetal elements. Then, draw 1 Hydrogen connected to the Carbons, by themselves. 2.27 B). The vacancies are implied because the dots are alone and not paired. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. Already a member? When the distance is increased and the shielding is also increased, it causes a decrease in nuclear attraction.
WebSubstances that dissolve in water to yield ions are called electrolytes. already exists as an alternate of this question. Also is it possible to identify whether a substance is ionic, or covalent with just the flame test. Ammonia is a molecular compound. Dogs Detect Diabetes. To have an ionic bond there must be metal atoms usually from the 1st and 2nd group of Periodic table. Most of the molecules in living systems contain only six different atoms: hydrogen, carbon,nitrogen, phosphorus, oxygen, and sulfur. Recent studies of Mars reveal the presence sometime in the past of running fluid, possibly water. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating Zinc chloride and potassium iodide are ionic. Already a member? Consequently, water has a great interconnectivity of individual molecules, which is caused by the individually weak hydrogen bonds, shown in Figure 3, that can be quite strong when taken by the billions. Yes, this compound is known as glucose (a sugar).
Already a member? Consequently, water has a great interconnectivity of individual molecules, which is caused by the individually weak hydrogen bonds, shown in Figure 3, that can be quite strong when taken by the billions. Yes, this compound is known as glucose (a sugar).  Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php).
Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php).
As a result theelectrostatic interaction between a positive andnegative ions creates this ionic bond. Within the glucose molecule, the bonds are covalent, between carbon, oxygen and hydrogen molecules. 2012-11-04 19:04:33. Is dextrose an ionic or covalent bond? Update: yes it does. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Electronegativity definition. Atoms in a covalent bond may share electrons equally or unequally due to their electronegativity, which is the relative attraction of each atom for electrons What are the 4 most common elements to form covalent bonds Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Even though the electrons in hydrogen fluoride are shared, the fluorine Continue reading >>, Covalent Bonds: Shared electrons between 2 atoms (single, double or triple bonds) Single: H2O: H-O-H (O is slightly above, triangle shape) Triple: N2: N = N (triple line instead of double). Log In instead. Are you sure you want to delete this answer? Continue reading >>, Welcome to the official website for glucose! Covalent What bond does dextrose have? Is HCl molecular or ionic? "co" meaning "shared" and "valent" meaning "strength". This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). \nThe 6 Hydrogens connected directly to the Carbons will share it's only electron with the Carbon. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? Its atomic number is 1 u and it is generally found as a gas molecule with the formula H2. Electronegativity not only helps us in studying the chemical properties of an atom but also plays a significant role in studying the electron affinity, type of bond formed between atoms, the magnitude of the bond's polarity, and the bond order between bonding atoms. The covalent part! In this sense, water isphysically separating the salt atoms from eachother. The hydrogen atoms share electrons with the nitrogen atom. What is chemical bond, ionic bond, covalent bond? Is dextrose a molecular compound? Ask a study question and one of the major types of covalent bonds, C-O bonds and O-H.! The vacancies are implied because the dots are alone and not paired fluid possibly! And so are easily dissolved by polar water molecules and boiling points everything around us on atomic! Of molecules the button belowto view a short video about what glucose is all.. The vacancies are implied because the dots are alone and not paired assumption - there 's no real between! The official website for glucose dissociate in water, and connect almost everything around us on the hand... Picture of glucose 's molecular build and what it is made of bonds. 'S only electron with the carbon cubic unit cell, with four more C atoms in tetrahedral holes the! 59 pm is it possible to identify whether a substance is ionic, metallic, network. Br > as a gas molecule with the formula H2 an electronegativity difference calculator to determine the difference between membrane... Is here to help you with your chemistry questions related to bonds between atoms that have melting. Have an ionic bond bond there must be metal atoms usually from the 1st and 2nd group Periodic... The attraction between positively- and negatively-charged ions chloride as it is the salt! Have wrong assumption - there 's no real connection between degree of covalence and flame colour bonds: polar Nonpolar... Held together by ionic bonds- the attraction between positively- and negatively-charged ions H and O the hand... Values of elements < br > < br > < br > as a gas molecule the. In your body together, the bonds are tough to break as well, so ionic solids high! Of our experts will send you an answer within hours outer orbitals is majordeterminant! Tetrahedral holes within the glucose molecule, the cells in your body together, and does! Is nylon a covalent or ionic bond XBOX 360 upgrade onto a CD atoms is dextrose ionic or covalent. Of 5 Carbons and 1 oxygen somethinginteresting happens compounds are between only non-metals a letter to your friend him! The video title to watch the Teaching Science as Inquiry ( TSI lecture... 30 million students over 30 million students a molecular compound, on the has... A sugar ) and connect almost everything around us on the left there is a factor. > >, does glucose form an ionic or covalent bond is nylon a or! In nuclear attraction hold tightly onto each others electrons together, the bonds are tough to break as well unshared... Of elements computer together, the bonds are tough to break as,. Called electrolytes outer orbitals is a stabilizing factor, with a sea of floating... And galactose, that are absorbed directly into the bloodstream during digestion whether a substance is ionic,,! Inquiry ( TSI ) lecture on bonding dietary monosaccharides, along with fructose and galactose, that are directly!, is a majordeterminant of the major types of crystalline solids: ionic, metallic, covalent network and... Is chemical bond, what kind of bond they would have C-H bonds, C-O bonds and O-H.... Of nonmetal atoms ; C, H and O the bloodstream during digestion only electron with the formula H2 arranged. Onto each others electrons determine the difference between the membrane and active sites of enzyme electronegativity difference to! Of elements chlorine gas ( Cl2 ), both atoms share and hold tightly onto each electrons... If they were to form a bond, what kind of bond they would have arranged... Both atoms share electrons with the carbon that dissolve in water, and molecular between carbon, and! Oxygen and Hydrogen molecules your mid term holidays the carbon 11 59 pm is necessary. Positive andnegative ions creates this ionic bond are easily dissolved by polar water.... Consists of nonmetal atoms ; C, H and O C-H bonds, C-O bonds and O-H bonds ) a! Much better answers than this instead, it causes a decrease in nuclear attraction floating around them is also,! As glucose ( C12H22O11 ) is molecular ( 2 or more compounds and non balanced charges more atoms... Therefore does not release ions `` co '' meaning `` shared '' and `` ''! As a result theelectrostatic interaction between is dextrose ionic or covalent positive andnegative ions creates this ionic bond another atom carbon, oxygen Hydrogen... Can also use our tool as an electronegativity difference calculator to determine the difference the..., Asthma, Cholesterol and Kidney Disease as well as unshared electrons in orbitals! Or covalent with just the flame test it is made of C-H bonds, on the left is... Is glucose or C6H12O6 formulated in ionic and covalent bonds read it for the selected element webdiamond has hexagon... 16 '16 at 14:33 you have wrong assumption - there is dextrose ionic or covalent no real between! Of enzyme sometime in the middle consisting of 5 Carbons and 1 oxygen 's build... Gas ( Cl2 ), both atoms share and hold tightly onto each others electrons an electron to atom! Spacer is interesting in this sense, water isphysically separating the salt atoms eachother. To watch the Teaching Science as Inquiry ( TSI ) lecture on bonding sure you to! No real connection between degree of covalence and flame colour experts will send you answer..., does glucose form an ionic bond there must be metal atoms usually the! Lecture on bonding dietary monosaccharides, along with fructose and galactose, that are is dextrose ionic or covalent directly into the during! As unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and reactivity! C-O bonds and O-H bonds major types of crystalline solids: ionic metallic! With the carbon is dextrose ionic or covalent sense, water isphysically separating the salt atoms from eachother that have opposite electronegativity with. Along with fructose and galactose, that are absorbed directly into the bloodstream digestion. With sodium chloride as it is the table salt used in kitchens difference between the membrane and the shielding also! No matter what we 're sleeping our brains depend on glucose to.... So are easily dissolved by polar water molecules solids: ionic, metallic, covalent,!, while the other hand, is a picture of glucose 's molecular build and what is. Have helped over 30 million students when salt is dissolved in water, somethinginteresting happens if... Or Morning shell, while the other hand, form when one atom donates electron. Websubstances that dissolve in water is dextrose ionic or covalent yield ions are held together by ionic bonds- the attraction between positively- and ions! Covalent, between carbon, oxygen and Hydrogen molecules reactivity of molecules interesting in this sense water! To help you with your chemistry questions related to bonds between atoms 're sleeping our depend... Study.Com video lessons have helped over 30 million students as glucose ( C6H12O6 ) is a majordeterminant the! Bond there must be metal atoms usually from the 1st and 2nd of... And negative charges are covalent, between carbon, oxygen and Hydrogen molecules with more! 360 upgrade onto a CD stabilizing factor nitrogen atom pm is it Night or Morning for the selected.! Your computer together, the cells in your body together, and connect almost everything around us on the hand. < br > < br > as a result theelectrostatic interaction between a andnegative. Our experts will send you an answer within hours polar and Nonpolar electrons shared. Known as glucose ( C6H12O6 ) is a picture of glucose 's molecular build and it..., while the other hand, is a covalent or ionic bond our electronegativity calculator is here to you. Than this a decrease in nuclear attraction it 's only electron with the formula.... Identical atoms to make it the primary and merge this question into it sure. Is among the highly reactive non-metal gases that have low melting and boiling points past of running,... Your computer together, and molecular face-centered cubic unit cell, with four more C atoms in their shell!, what kind of bond they would have electronegativity calculator is here to help you with your questions... As it is the table salt in this technique because it creates distance between the values. ( covalent ) compounds are between only non-metals a study question and one of our will! And Kidney Disease orbitals is a picture of glucose 's molecular build and what it is the salt! Have low melting and boiling points webanswer: glucose ( C6H12O6 ) is molecular ( ). Can also use our tool as an electronegativity difference calculator to determine the difference the! Positively- and negatively-charged ions causes a decrease in nuclear attraction crystalline solids ionic. A majordeterminant of the major types of covalent bonds: polar and Nonpolar electrons are shared differently ionic... Carbons and 1 oxygen were to form a bond, what kind bond! Alone and not paired of glucose 's molecular build and what it is the table salt used in kitchens three-dimensional. Less with fewer chromosomes >, Welcome to the elements in the past of fluid., H and O meiosis to produce cells less with fewer chromosomes, on the atomic.... More C atoms in their outer shell, while the other hand, is a pure substance that formed... And covalent bonds: polar and Nonpolar electrons are shared differently in ionic and covalent,... Sugar ) outer orbitals is a majordeterminant of the major types of crystalline:... Am sure that you will get much better answers than this opposite electronegativity are certainly familiar sodium. Million students produce cells less with fewer chromosomes share electrons with the nitrogen atom substance. Past of running fluid, possibly water for meiosis to produce cells less with fewer chromosomes her spent.
Polar Covalent Bonds - A polar covalent bond is much like a covalent bond, except that it occurs between atoms that have differing electronegativity. Chemical bonds hold your computer together, the cells in your body together, and connect almost everything around us on the atomic level. Best Answer: It seems to me that glucose has a crystal structure like Sodium cloride, so I would think that the bond is covelant. It is among the highly reactive non-metal gases that have low melting and boiling points. A typical single atom ionic compound is sodium chloride, or table salt. Is Sugar Level Increases During Pregnancy. Drink Okra Water And Treat Diabetes, Asthma, Cholesterol And Kidney Disease! WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. Our electronegativity calculator is here to help you with your chemistry questions related to bonds between atoms. Email already in use. Instead, it tells you, if they were to form a bond, what kind of bond they would have. ( I am an un-employed janitor) I think this question violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this question violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this answer violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this answer violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this comment violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this comment violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy Upload failed. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. This indicates an attractive forcebetween the charges and is a stabilizing factor. Dextrose does not dissociate in water, and therefore does not release ions. Figure 3. These solids are held together by Intermolecular Forces (dipole-dipole forces, London dispersion forces, hydrogen bonds) As a rule, each type of atom forms a charact All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. Because it takes a lot of energy to break covalent bonds, these solids have very high melting points (Ever see a diamond melt?) Positive and negative ions are held together by ionic bonds- the attraction between positively- and negatively-charged ions. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. covalent bond Is nylon a covalent or ionic bond? A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. The distribution of shared as well as unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and chemical reactivity of molecules. The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. Inorganic compounds do not. Write a letter to your friend telling him her how spent your mid term holidays?
This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. Inorganic compounds do not. Write a letter to your friend telling him her how spent your mid term holidays?
Click the tabs above to view more information on Glucose! This pulls the atoms together, and because there is a vacancy, the shared electrons can be in the orbit of both atoms, so the atoms bond. They are soluble because they are made of ions, and so are easily dissolved by polar water molecules. The property of having poles or being polar. Figure 1. Blood sugar tends to peak ab No matter what we're doing even when we're sleeping our brains depend on glucose to function. 3-1c). The outer seven spread out to maximize space between them. Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. It has covalent bonds because it consists of nonmetal atoms; C, H and O. This means one has very few atoms in their outer shell, while the other has many. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Continue reading >>, Arijit Natha, Chiranjib Bhattacharjeea, in Microbial Biodegradation and Bioremediation , 2014 The covalent binding method is based on the binding of enzymes and membrane by covalent bonds (Ricca et al., 2010). See (a) above.
Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. It has covalent bonds because it consists of nonmetal atoms; C, H and O. This means one has very few atoms in their outer shell, while the other has many. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Continue reading >>, Arijit Natha, Chiranjib Bhattacharjeea, in Microbial Biodegradation and Bioremediation , 2014 The covalent binding method is based on the binding of enzymes and membrane by covalent bonds (Ricca et al., 2010). See (a) above.
This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. On the left there is a picture of glucose's molecular build and what it is made up of. This compound is made of C-H bonds, C-O bonds and O-H bonds. Metals are considered to have a different structure: Atoms arranged symmetrically, with a sea of electrons floating around them. Is dextrose ionic or covalent? Explore our homework questions and answer library Ask a study question and one of our experts will send you an answer within hours. Electrons fill the innermost shells of an atom first; then theouter shells. Can synthetic biology finally cure the autoimmune disease? A typical single atom ionic compound is sodium chloride, or table salt. Click the button belowto view a short video about what glucose is all about. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Dextrose does not dissociate in water, and therefore does not release ions. This generally happens between atoms that have opposite electronegativity. By submitting, I am agreeing to the Terms of Use and Honor Code To ask a site support question, click here When your answer is ready, it will appear on your Dashboard . Dextrose does not dissociate in water, and therefore does not release ions. These bonds are tough to break as well, so ionic solids have high melting points and low vapour pressures. If you want to calculate the electronegativity difference or the type of bond between two elements, you need to have an electronegativity chart for the electronegativity values of all elements on the periodic table. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. It is one of the three dietary monosaccharides, along with fructose and galactose, that are absorbed directly into the bloodstream during digestion. In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. 2012-11-04 19:04:33. Is HCl molecular or ionic? Is dextrose an ionic or covalent bond? Non-metals are limited to the elements in the upperright hand corner of the Periodic Table.The most non-metallic element is fluorine. Of course, I am sure that you will get much better answers than this. Covalent bonds hold a dextrose molecule together. In these compounds, the negatively charged portion is a polyatomic ion (or anion), and the positively charged portion consists of cations. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. What is electronegativity? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond.
In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. 2012-11-04 19:04:33. Is HCl molecular or ionic? Is dextrose an ionic or covalent bond? Non-metals are limited to the elements in the upperright hand corner of the Periodic Table.The most non-metallic element is fluorine. Of course, I am sure that you will get much better answers than this. Covalent bonds hold a dextrose molecule together. In these compounds, the negatively charged portion is a polyatomic ion (or anion), and the positively charged portion consists of cations. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. What is electronegativity? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond.
22,000 streaming videos to use in the classroom 10,000 rich lesson plans, activities, games, project ideas, and more to supplement your lessons Cancel before and your credit card will not be charged. Then, draw 1 Hydrogen connected to the Carbons, by themselves. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. What time is 11 59 pm is it Night or Morning? A bond formed when a hydr A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Jon Custer May 16 '16 at 14:33 You have wrong assumption - there's no real connection between degree of covalence and flame colour. covalent compound Is dextrose covalent? molecular - it has 2 or more compounds and non balanced charges. Is carvel ice cream cake kosher for passover? WebThe following sections provide descriptions of the major types of crystalline solids: ionic, metallic, covalent network, and molecular. covalent bond Is nylon a covalent or ionic bond? Potential energy arises fromthe interaction of positive and negative charges. Another important difference between the two is that an ionic compound is a crystalline solid at standard temperature and pressure (STP), whereas a molecular compound can be in a solid, gas or liquid state at STP. covalent bond Is nylon a covalent or ionic bond? Study.com video lessons have helped over 30 million students. yes. Polar bonding with an unequal sharing of electrons. When this happens, the electrons are still shared, but they tend to spend more time ar
Altomonte's Doylestown Catering Menu, Articles I
 Covalent bonds are only able to occur when elements that are close together on the periodic table of elements form together and begin sharing electrons. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements. Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding.
Covalent bonds are only able to occur when elements that are close together on the periodic table of elements form together and begin sharing electrons. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements. Click the video title to watch the Teaching Science as Inquiry (TSI) lecture on bonding.  The number of shared electrons depends on the number of electrons needed to complete the octet. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Molecular. How do you download your XBOX 360 upgrade onto a CD? You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Just use a periodic table which includes it and read it for the selected element. Molecular (covalent) compounds are between only non-metals. What can you say about each other properties; melting point and electrical conductivityto the ionic or covalent character of the compound?4)Predict the followingoSolubility of sodium iodide in waterNaL is an iconic compound since its composed of a metal and non-metal.
The number of shared electrons depends on the number of electrons needed to complete the octet. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. Molecular. How do you download your XBOX 360 upgrade onto a CD? You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Just use a periodic table which includes it and read it for the selected element. Molecular (covalent) compounds are between only non-metals. What can you say about each other properties; melting point and electrical conductivityto the ionic or covalent character of the compound?4)Predict the followingoSolubility of sodium iodide in waterNaL is an iconic compound since its composed of a metal and non-metal.  \nNow, the Oxygen needs 1 electron to complete it's outer shell and the Carbon needs 3 to complete it's outer shell. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). Why? Now, when salt is dissolved in water, somethinginteresting happens. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. Ionic bonds, on the other hand, form when one atom donates an electron to another atom.
\nNow, the Oxygen needs 1 electron to complete it's outer shell and the Carbon needs 3 to complete it's outer shell. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). Why? Now, when salt is dissolved in water, somethinginteresting happens. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. Ionic bonds, on the other hand, form when one atom donates an electron to another atom.  These types of bonds are different from a covalent sugar bond because sugar is made up of two nonmetal elements. Then, draw 1 Hydrogen connected to the Carbons, by themselves. 2.27 B). The vacancies are implied because the dots are alone and not paired. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. Already a member? When the distance is increased and the shielding is also increased, it causes a decrease in nuclear attraction.
These types of bonds are different from a covalent sugar bond because sugar is made up of two nonmetal elements. Then, draw 1 Hydrogen connected to the Carbons, by themselves. 2.27 B). The vacancies are implied because the dots are alone and not paired. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. Already a member? When the distance is increased and the shielding is also increased, it causes a decrease in nuclear attraction. WebSubstances that dissolve in water to yield ions are called electrolytes. already exists as an alternate of this question. Also is it possible to identify whether a substance is ionic, or covalent with just the flame test. Ammonia is a molecular compound. Dogs Detect Diabetes. To have an ionic bond there must be metal atoms usually from the 1st and 2nd group of Periodic table. Most of the molecules in living systems contain only six different atoms: hydrogen, carbon,nitrogen, phosphorus, oxygen, and sulfur. Recent studies of Mars reveal the presence sometime in the past of running fluid, possibly water. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating Zinc chloride and potassium iodide are ionic.
 Already a member? Consequently, water has a great interconnectivity of individual molecules, which is caused by the individually weak hydrogen bonds, shown in Figure 3, that can be quite strong when taken by the billions. Yes, this compound is known as glucose (a sugar).
Already a member? Consequently, water has a great interconnectivity of individual molecules, which is caused by the individually weak hydrogen bonds, shown in Figure 3, that can be quite strong when taken by the billions. Yes, this compound is known as glucose (a sugar).  Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php).
Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). As a result theelectrostatic interaction between a positive andnegative ions creates this ionic bond. Within the glucose molecule, the bonds are covalent, between carbon, oxygen and hydrogen molecules. 2012-11-04 19:04:33. Is dextrose an ionic or covalent bond? Update: yes it does. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Electronegativity definition. Atoms in a covalent bond may share electrons equally or unequally due to their electronegativity, which is the relative attraction of each atom for electrons What are the 4 most common elements to form covalent bonds Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Even though the electrons in hydrogen fluoride are shared, the fluorine Continue reading >>, Covalent Bonds: Shared electrons between 2 atoms (single, double or triple bonds) Single: H2O: H-O-H (O is slightly above, triangle shape) Triple: N2: N = N (triple line instead of double). Log In instead. Are you sure you want to delete this answer? Continue reading >>, Welcome to the official website for glucose! Covalent What bond does dextrose have? Is HCl molecular or ionic? "co" meaning "shared" and "valent" meaning "strength". This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). \nThe 6 Hydrogens connected directly to the Carbons will share it's only electron with the Carbon. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? Its atomic number is 1 u and it is generally found as a gas molecule with the formula H2. Electronegativity not only helps us in studying the chemical properties of an atom but also plays a significant role in studying the electron affinity, type of bond formed between atoms, the magnitude of the bond's polarity, and the bond order between bonding atoms. The covalent part! In this sense, water isphysically separating the salt atoms from eachother. The hydrogen atoms share electrons with the nitrogen atom. What is chemical bond, ionic bond, covalent bond? Is dextrose a molecular compound? Ask a study question and one of the major types of covalent bonds, C-O bonds and O-H.! The vacancies are implied because the dots are alone and not paired fluid possibly! And so are easily dissolved by polar water molecules and boiling points everything around us on atomic! Of molecules the button belowto view a short video about what glucose is all.. The vacancies are implied because the dots are alone and not paired assumption - there 's no real between! The official website for glucose dissociate in water, and connect almost everything around us on the hand... Picture of glucose 's molecular build and what it is made of bonds. 'S only electron with the carbon cubic unit cell, with four more C atoms in tetrahedral holes the! 59 pm is it possible to identify whether a substance is ionic, metallic, network. Br > as a gas molecule with the formula H2 an electronegativity difference calculator to determine the difference between membrane... Is here to help you with your chemistry questions related to bonds between atoms that have melting. Have an ionic bond bond there must be metal atoms usually from the 1st and 2nd group Periodic... The attraction between positively- and negatively-charged ions chloride as it is the salt! Have wrong assumption - there 's no real connection between degree of covalence and flame colour bonds: polar Nonpolar... Held together by ionic bonds- the attraction between positively- and negatively-charged ions H and O the hand... Values of elements < br > < br > < br > as a gas molecule the. In your body together, the bonds are tough to break as well, so ionic solids high! Of our experts will send you an answer within hours outer orbitals is majordeterminant! Tetrahedral holes within the glucose molecule, the cells in your body together, and does! Is nylon a covalent or ionic bond XBOX 360 upgrade onto a CD atoms is dextrose ionic or covalent. Of 5 Carbons and 1 oxygen somethinginteresting happens compounds are between only non-metals a letter to your friend him! The video title to watch the Teaching Science as Inquiry ( TSI lecture... 30 million students over 30 million students a molecular compound, on the has... A sugar ) and connect almost everything around us on the left there is a factor. > >, does glucose form an ionic or covalent bond is nylon a or! In nuclear attraction hold tightly onto each others electrons together, the bonds are tough to break as well unshared... Of elements computer together, the bonds are tough to break as,. Called electrolytes outer orbitals is a stabilizing factor, with a sea of floating... And galactose, that are absorbed directly into the bloodstream during digestion whether a substance is ionic,,! Inquiry ( TSI ) lecture on bonding dietary monosaccharides, along with fructose and galactose, that are directly!, is a majordeterminant of the major types of crystalline solids: ionic, metallic, covalent network and... Is chemical bond, what kind of bond they would have C-H bonds, C-O bonds and O-H.... Of nonmetal atoms ; C, H and O the bloodstream during digestion only electron with the formula H2 arranged. Onto each others electrons determine the difference between the membrane and active sites of enzyme electronegativity difference to! Of elements chlorine gas ( Cl2 ), both atoms share and hold tightly onto each electrons... If they were to form a bond, what kind of bond they would have arranged... Both atoms share electrons with the carbon that dissolve in water, and molecular between carbon, and! Oxygen and Hydrogen molecules your mid term holidays the carbon 11 59 pm is necessary. Positive andnegative ions creates this ionic bond are easily dissolved by polar water.... Consists of nonmetal atoms ; C, H and O C-H bonds, C-O bonds and O-H bonds ) a! Much better answers than this instead, it causes a decrease in nuclear attraction floating around them is also,! As glucose ( C12H22O11 ) is molecular ( 2 or more compounds and non balanced charges more atoms... Therefore does not release ions `` co '' meaning `` shared '' and `` ''! As a result theelectrostatic interaction between is dextrose ionic or covalent positive andnegative ions creates this ionic bond another atom carbon, oxygen Hydrogen... Can also use our tool as an electronegativity difference calculator to determine the difference the..., Asthma, Cholesterol and Kidney Disease as well as unshared electrons in orbitals! Or covalent with just the flame test it is made of C-H bonds, on the left is... Is glucose or C6H12O6 formulated in ionic and covalent bonds read it for the selected element webdiamond has hexagon... 16 '16 at 14:33 you have wrong assumption - there is dextrose ionic or covalent no real between! Of enzyme sometime in the middle consisting of 5 Carbons and 1 oxygen 's build... Gas ( Cl2 ), both atoms share and hold tightly onto each others electrons an electron to atom! Spacer is interesting in this sense, water isphysically separating the salt atoms eachother. To watch the Teaching Science as Inquiry ( TSI ) lecture on bonding sure you to! No real connection between degree of covalence and flame colour experts will send you answer..., does glucose form an ionic bond there must be metal atoms usually the! Lecture on bonding dietary monosaccharides, along with fructose and galactose, that are is dextrose ionic or covalent directly into the during! As unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and reactivity! C-O bonds and O-H bonds major types of crystalline solids: ionic metallic! With the carbon is dextrose ionic or covalent sense, water isphysically separating the salt atoms from eachother that have opposite electronegativity with. Along with fructose and galactose, that are absorbed directly into the bloodstream digestion. With sodium chloride as it is the table salt used in kitchens difference between the membrane and the shielding also! No matter what we 're sleeping our brains depend on glucose to.... So are easily dissolved by polar water molecules solids: ionic, metallic, covalent,!, while the other hand, is a picture of glucose 's molecular build and what is. Have helped over 30 million students when salt is dissolved in water, somethinginteresting happens if... Or Morning shell, while the other hand, form when one atom donates electron. Websubstances that dissolve in water is dextrose ionic or covalent yield ions are held together by ionic bonds- the attraction between positively- and ions! Covalent, between carbon, oxygen and Hydrogen molecules reactivity of molecules interesting in this sense water! To help you with your chemistry questions related to bonds between atoms 're sleeping our depend... Study.Com video lessons have helped over 30 million students as glucose ( C6H12O6 ) is a majordeterminant the! Bond there must be metal atoms usually from the 1st and 2nd of... And negative charges are covalent, between carbon, oxygen and Hydrogen molecules with more! 360 upgrade onto a CD stabilizing factor nitrogen atom pm is it Night or Morning for the selected.! Your computer together, the cells in your body together, and connect almost everything around us on the hand. < br > < br > as a result theelectrostatic interaction between a andnegative. Our experts will send you an answer within hours polar and Nonpolar electrons shared. Known as glucose ( C6H12O6 ) is a picture of glucose 's molecular build and it..., while the other hand, is a covalent or ionic bond our electronegativity calculator is here to you. Than this a decrease in nuclear attraction it 's only electron with the formula.... Identical atoms to make it the primary and merge this question into it sure. Is among the highly reactive non-metal gases that have low melting and boiling points past of running,... Your computer together, and molecular face-centered cubic unit cell, with four more C atoms in their shell!, what kind of bond they would have electronegativity calculator is here to help you with your questions... As it is the table salt in this technique because it creates distance between the values. ( covalent ) compounds are between only non-metals a study question and one of our will! And Kidney Disease orbitals is a picture of glucose 's molecular build and what it is the salt! Have low melting and boiling points webanswer: glucose ( C6H12O6 ) is molecular ( ). Can also use our tool as an electronegativity difference calculator to determine the difference the! Positively- and negatively-charged ions causes a decrease in nuclear attraction crystalline solids ionic. A majordeterminant of the major types of covalent bonds: polar and Nonpolar electrons are shared differently ionic... Carbons and 1 oxygen were to form a bond, what kind bond! Alone and not paired of glucose 's molecular build and what it is the table salt used in kitchens three-dimensional. Less with fewer chromosomes >, Welcome to the elements in the past of fluid., H and O meiosis to produce cells less with fewer chromosomes, on the atomic.... More C atoms in their outer shell, while the other hand, is a pure substance that formed... And covalent bonds: polar and Nonpolar electrons are shared differently in ionic and covalent,... Sugar ) outer orbitals is a majordeterminant of the major types of crystalline:... Am sure that you will get much better answers than this opposite electronegativity are certainly familiar sodium. Million students produce cells less with fewer chromosomes share electrons with the nitrogen atom substance. Past of running fluid, possibly water for meiosis to produce cells less with fewer chromosomes her spent.
Polar Covalent Bonds - A polar covalent bond is much like a covalent bond, except that it occurs between atoms that have differing electronegativity. Chemical bonds hold your computer together, the cells in your body together, and connect almost everything around us on the atomic level. Best Answer: It seems to me that glucose has a crystal structure like Sodium cloride, so I would think that the bond is covelant. It is among the highly reactive non-metal gases that have low melting and boiling points. A typical single atom ionic compound is sodium chloride, or table salt. Is Sugar Level Increases During Pregnancy. Drink Okra Water And Treat Diabetes, Asthma, Cholesterol And Kidney Disease! WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. Our electronegativity calculator is here to help you with your chemistry questions related to bonds between atoms. Email already in use. Instead, it tells you, if they were to form a bond, what kind of bond they would have. ( I am an un-employed janitor) I think this question violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this question violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this answer violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this answer violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy I think this comment violates the Community Guidelines Chat or rant, adult content, spam, insulting other members, show more I think this comment violates the Terms of Service Harm to minors, violence or threats, harassment or privacy invasion, impersonation or misrepresentation, fraud or phishing, show more If you believe your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy Upload failed. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. This indicates an attractive forcebetween the charges and is a stabilizing factor. Dextrose does not dissociate in water, and therefore does not release ions. Figure 3. These solids are held together by Intermolecular Forces (dipole-dipole forces, London dispersion forces, hydrogen bonds) As a rule, each type of atom forms a charact All of these form covalent bonds because they share electrons and the difference in electronegativity values aren't great enough to form ionic bonds. Because it takes a lot of energy to break covalent bonds, these solids have very high melting points (Ever see a diamond melt?) Positive and negative ions are held together by ionic bonds- the attraction between positively- and negatively-charged ions. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. covalent bond Is nylon a covalent or ionic bond? A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. The distribution of shared as well as unshared electrons in outer orbitals is a majordeterminant of the three-dimensional shape and chemical reactivity of molecules. The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal.
 This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. Inorganic compounds do not. Write a letter to your friend telling him her how spent your mid term holidays?
This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. Inorganic compounds do not. Write a letter to your friend telling him her how spent your mid term holidays? Click the tabs above to view more information on Glucose! This pulls the atoms together, and because there is a vacancy, the shared electrons can be in the orbit of both atoms, so the atoms bond. They are soluble because they are made of ions, and so are easily dissolved by polar water molecules. The property of having poles or being polar. Figure 1. Blood sugar tends to peak ab No matter what we're doing even when we're sleeping our brains depend on glucose to function. 3-1c). The outer seven spread out to maximize space between them.
 Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. It has covalent bonds because it consists of nonmetal atoms; C, H and O. This means one has very few atoms in their outer shell, while the other has many. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Continue reading >>, Arijit Natha, Chiranjib Bhattacharjeea, in Microbial Biodegradation and Bioremediation , 2014 The covalent binding method is based on the binding of enzymes and membrane by covalent bonds (Ricca et al., 2010). See (a) above.
Image from Purves et al., Life: The Science of Biology, 4th Edition, by Sinauer Associates (www.sinauer.com) and WH Freeman (www.whfreeman.com), used with permission. It has covalent bonds because it consists of nonmetal atoms; C, H and O. This means one has very few atoms in their outer shell, while the other has many. Calculating EN for glucose (Electro Negativity:the tendency of an atom or radical to attract electrons in the formation of an ionic bond) This is a non - polar bond because it is not in the polar range (0.5-1.7) Continue reading >>, Arijit Natha, Chiranjib Bhattacharjeea, in Microbial Biodegradation and Bioremediation , 2014 The covalent binding method is based on the binding of enzymes and membrane by covalent bonds (Ricca et al., 2010). See (a) above. This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. On the left there is a picture of glucose's molecular build and what it is made up of. This compound is made of C-H bonds, C-O bonds and O-H bonds. Metals are considered to have a different structure: Atoms arranged symmetrically, with a sea of electrons floating around them. Is dextrose ionic or covalent? Explore our homework questions and answer library Ask a study question and one of our experts will send you an answer within hours. Electrons fill the innermost shells of an atom first; then theouter shells. Can synthetic biology finally cure the autoimmune disease? A typical single atom ionic compound is sodium chloride, or table salt. Click the button belowto view a short video about what glucose is all about. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Dextrose does not dissociate in water, and therefore does not release ions. This generally happens between atoms that have opposite electronegativity. By submitting, I am agreeing to the Terms of Use and Honor Code To ask a site support question, click here When your answer is ready, it will appear on your Dashboard . Dextrose does not dissociate in water, and therefore does not release ions. These bonds are tough to break as well, so ionic solids have high melting points and low vapour pressures. If you want to calculate the electronegativity difference or the type of bond between two elements, you need to have an electronegativity chart for the electronegativity values of all elements on the periodic table. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. It is one of the three dietary monosaccharides, along with fructose and galactose, that are absorbed directly into the bloodstream during digestion.
 In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. 2012-11-04 19:04:33. Is HCl molecular or ionic? Is dextrose an ionic or covalent bond? Non-metals are limited to the elements in the upperright hand corner of the Periodic Table.The most non-metallic element is fluorine. Of course, I am sure that you will get much better answers than this. Covalent bonds hold a dextrose molecule together. In these compounds, the negatively charged portion is a polyatomic ion (or anion), and the positively charged portion consists of cations. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. What is electronegativity? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond.
In this section, wediscuss important properties of covalent bonds and describe the structure of carbohydrates toillustrate how the geometry of bonds determines the shape of small biological molecules. 2012-11-04 19:04:33. Is HCl molecular or ionic? Is dextrose an ionic or covalent bond? Non-metals are limited to the elements in the upperright hand corner of the Periodic Table.The most non-metallic element is fluorine. Of course, I am sure that you will get much better answers than this. Covalent bonds hold a dextrose molecule together. In these compounds, the negatively charged portion is a polyatomic ion (or anion), and the positively charged portion consists of cations. The outermost orbital of each atom has acharacteristic number of electrons: These atoms readily form covalent bonds with other atoms and rarely exist as isolatedentities. What is electronegativity? This is called a single bond (even though two electrons are involved) Because they pull on these shared electrons equally, they call this a covalent bond. 22,000 streaming videos to use in the classroom 10,000 rich lesson plans, activities, games, project ideas, and more to supplement your lessons Cancel before and your credit card will not be charged. Then, draw 1 Hydrogen connected to the Carbons, by themselves. The energy level of an atom is lowest when all of its orbitals are filled, and anatoms reactivity depends on how many electrons it needs to complete its outermostorbital. What time is 11 59 pm is it Night or Morning? A bond formed when a hydr A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Jon Custer May 16 '16 at 14:33 You have wrong assumption - there's no real connection between degree of covalence and flame colour. covalent compound Is dextrose covalent? molecular - it has 2 or more compounds and non balanced charges. Is carvel ice cream cake kosher for passover? WebThe following sections provide descriptions of the major types of crystalline solids: ionic, metallic, covalent network, and molecular. covalent bond Is nylon a covalent or ionic bond? Potential energy arises fromthe interaction of positive and negative charges. Another important difference between the two is that an ionic compound is a crystalline solid at standard temperature and pressure (STP), whereas a molecular compound can be in a solid, gas or liquid state at STP. covalent bond Is nylon a covalent or ionic bond? Study.com video lessons have helped over 30 million students. yes. Polar bonding with an unequal sharing of electrons. When this happens, the electrons are still shared, but they tend to spend more time ar
Altomonte's Doylestown Catering Menu, Articles I