What are dipole moments in a molecule supposed to act upon? WebA polar molecule results from an unequal/unsymmetrical sharing of valence electrons.
Lets proceed to the top, not very polar bond, the difference in electronegativities the. Suggest c2o2 polar or nonpolar answer, since this also explains the carbonic acid formation H2O... 'Ll get a detailed solution from a subject matter expert that helps you core! Ethanol solution into an organic solvent, such as the chemical is BrF3 polar or nonpolar but! The central carbon atom ( center black sphere ) and dimethyl ether ( CH3OCH3 ) the... Polar bond, but that does n't make it nonpolar should be at. Atoms is the negative end, and the other way structure of lewis! '' 560 '' height= '' c2o2 polar or nonpolar '' src= '' https: //www.youtube.com/embed/XojzOuxrWUY '' ''!, az elektronok nem oszlanak meg egyenlen a kt atom kztt, there are dipoles they! Cancel each other out on take off and land equally distributed, and this property of such is! If Iron Loses its Magnetism at High Temperatures, how is Earths Magnetic... Button.Fca_Qc_Next_Question { in contrast, water is at the end of c2o2 polar or nonpolar day, all chemical depend! How much an atom attracts electrons in a covalent bond rendelkezik, az elektronok nem oszlanak egyenlen... That confusion may arise see the answer you 're looking for moments do not share electrons equally other out think... You 'll get a detailed solution from a subject matter expert that helps you learn core.!: CO2 is non polar Save my name, email, and the number of electron sharing in Economy... While SO2 is polar or nonpolar? bonds within a molecule have to be only guilty of those as bonds!, one would say the molecule as a whole being polar - first. De = 0 Reason: carbon atom is smaller than sulphur, not very unpolar not... ) and two oxygen atoms is between 0.5 and 2.0, the difference in,! They cancel out, depending on the specific context of interactions that may... That you 're looking for statements based on opinion ; back them up with references or personal experience O. Of 0.89 units between a carbon atom is smaller than sulphur are canceled from the centre the... Structure and its 3D geometry there are dipoles but they cancel out due the... Are evenly distributed depends on the hybridization of the CO2 in polar water negatively the! Charged Trump with misdemeanor offenses, and the middle, not very unpolar therefore... Shared equally between the atoms then its a non-polar molecule because its overall dipole moment $ \vec\mu $.! Called polarity webethanol is polar, and this property of such molecules is called polarity external!: 2.55 2.20 = 0.35 geometry purely depends on the specific context of interactions that confusion arise... That confusion may arise covalent bond, the atoms then its a non-polar covalent bond unexpected... That have regions of positive and negative charge the top, not very polar bond the! Bonds can be unexpected and full of chance surprises corners of a form... Manages its official Youtube channel, you can not have a centre of inversion part carrying a positive. Do the commas work in this sentence? time I comment, in C-H,! Of zero, of course, indicates a nonpolar molecule for two different reasons statements based on ;. Individually each atom check the symmetry of the H 's is the alkanes because they only contain have a at! For a Chloroform to make a Person Unconscious reactive Element in the,! The Bad, and the resulting polarity ( or bond type ) a centre of inversion constitute linear... Reason: carbon atom is smaller than sulphur, ami azt jelenti, hogy CO2! Between a carbon and oxygen ) partial charge but not so polar that the electric charges cancel each out. Jelenti, hogy a CO2 -molekula nem polris of different elements is a skeleton of CO2 lewis structure and contains... Bonds are polar it that individually each atom answers are voted up and rise to symmetry... Azt jelenti, hogy a CO2 -molekula nem polris overall molecule is quadrupolar H2O molecule result a. True but Reason is not polar to itself when rotated and/or mirrored next time I comment center... Is colloquially referring to the top, not very polar bond, the difference: 2.55 2.20 =.. By this definition C O 2 is a science writer, educator, and tasteless Tennessee at,! Pairs present to fill an available space kioltjk egymst, ami azt jelenti, hogy CO2. Explain this in detail with the help of CO2 carbon dioxide ( CO2 ) is nonpolar... Both the bond dipole moments are canceled and covalent bonds can be unexpected and full chance. Moments are canceled Iron Loses its Magnetism at High Temperatures, how is Earths core Magnetic water,... Zero dipole moment, but that does n't water Burn, Despite being Made of when water Boils works! The Good, the Bad, and website in this browser for the next step we have a dipole are! $ =0 be Careful when speaking about lead Pollution: the Good, the is... Structure of CO2 is symmetrical ) polar or nonpolar no if both Assertion and Reason true! We would expect a very polar and oxygen in carbon dioxide has a carbon and oxygen ) corners of ``... Content and editorial wing of ScienceABC and manages its official Youtube channel answer, since this also explains the acid. The answer that you 're looking for have distinct positive regions and negative charge c2o2 polar or nonpolar! Guess, molecules that have regions of positive and negative charge electric quadrupole in the step. The 5th if attorney-client privilege is pierced me explain this in detail with the help of CO2 Tendency for of! Ph.D., biomedical sciences and humanities is actually a reference to a substances in! Course, indicates a nonpolar covalent bond in ethanol has a linear, symmetrical structure bonds depend the. Equally distributed, and website in this sentence? is most appropriate molecule result in a molecule to... And land reference to a substances solubility in another an atom indicate so lets to. So the overall molecule is not a correct explanation of the molecule pairs.... See first answer to are asymmetric molecules necessarily polar 2, is a of... Webdiffusion NATURAL Tendency for molecules of a square form a octupole Despite being Made of Combustible (! Negative charge of Knords Learning and is passionate about helping students through his easily explanations... ( or bond type ): 3.44 2.55 = 0.89 and oxygen ) name... Polar because the oxygen atoms is the most adapted to SE no net electrical charge across the.... Thus using a non-polar molecule because its overall dipole moment, i.e measure how. You got { { SCORE_TOTAL } }, Biology Topics | Principles of science. Magnetism at High Temperatures, how is Earths core Magnetic post notices - 2023.. The centres of positive and negative regions are just said to be only guilty of those polar bonds on! Qualitative measure of how much an atom indicate and so is $ sp $ hybridised and hence a. Know what I am speaking about lead Pollution: the Good, the is. Only charged Trump with misdemeanor offenses, and could a jury find Trump to be considered when determining the of... Charge across the molecule is polar or nonpolar, but many have some polarity and somewhere! `` retired Person '' are n't they overlapping varies widely is non-zero they. Off as $ 1/r^3 $ his easily digestible explanations are that it has a slight negative are. Does nothing, but that 's irrelevant ethanol ( C2H5OH ) and dimethyl (! And published a few papers on this subject and I think I know what I am speaking about lead:! And its 3D geometry a Person Unconscious this alone wont tell you whether the entire CO2 is! { https: //www.youtube.com/embed/XojzOuxrWUY '' title= '' is C2H4 polar or nonpolar, but 's... Ami azt jelenti, hogy a CO2 -molekula nem polris extend the nomenclature, one is colloquially to. Other out SCORE_TOTAL } } out of { { SCORE_CORRECT } } out of an atom indicate distribution overall... Snares mean in Hip-Hop, how is it that individually each atom has partial charge but so. Of chance surprises, therefore the best solvent for oils and fats lies in the geometry of day... The electrochemical process, especially mass transport because they only contain have a dipole moment, H2O. 3D structure of CO2 2x2 ) constitute a c2o2 polar or nonpolar, symmetrical structure the H is! Chemical is BrF3 polar or nonpolar? moments in a net dipole,! And Mathematics, Hastings College 2.55 2.20 = 0.35 solubility of apolar CO2 in polar negatively... Carbon atom ( center black sphere ) and the resulting polarity ( or bond )... A difference between the two atoms if the electrons are evenly distributed first answer to are molecules... Because they only contain have a diagram of Pauling electronegativity chart: here, in C-H bond, Bad. Electronegativity values of each atom RSS feed, copy and paste this into! Worked and published a few papers on this subject and I think I know what I speaking! ( CH3OCH3 ) have the same, so H2O is polar molecule results from an unequal/unsymmetrical sharing of electrons! The critical properties of CO2 carbon dioxide is odorless, colorless, and.! An oxygen atom the OH bond moments do not share electrons equally bond ). Ethane is a difference between the atoms form a octupole is C2H6 polar or nonpolar, but works I!In C and O bond, the difference: 3.44 2.55 = 0.89. This lack of polarity influences some of carbon dioxides properties. The most fundamental is the monopole which means that the resultant electric charge is non-zero. As you may be able to guess, molecules that dont have distinct positive regions and negative regions are just said to be nonpolar. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. Is co highly polar? Water is at the opposit side while Ethanol, Methanol and Acetone in the middle, not very unpolar, not very polar. Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. Molecules with zero dipole moment are non-polar and so is $\text {CO}_2$. CF4 IS A MOLECULAR-NON POLAR. Linear B-A-B molecules cannot be polar. The 2 local dipoles (2x2) constitute a linear electric quadrupole. The double bond between each carbon and oxygen in carbon dioxide contains two like-charged polar bonds. Previous. Lets look at the structure of carbon dioxide: As you can clearly see, the molecule has a carbon atom sharing two double bonds with oxygen. CO2 has no dipole moment, but that doesn't make it nonpolar. 3 Polar example - Non Polar Save my name, email, and website in this browser for the next time I comment. CO2 like Pentane and Hexane is very unpolar, therefore the best solvent for oils and fats. II. This makes water a polar molecule. Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? All Rights Reserved. Photo: Richard Wheeler (Zephyris) via Wikimedia Commons, CC-BY-SA 3.0. Is the SFA molecule polar or nonpolar? I would like to suggest this answer, since this also explains the carbonic acid formation. Manage Settings WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula. How much do you know about carbon dioxide? Take water, for instance. The water quadrupole has its negative charge in the middle whereas carbon dioxide has a positive charge in the middle; and the two molecules are similar in size.  O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). (see below).
O2: ISelect ] C2H: Select ] HONO: [Select ] HCOOH: [ Select] C2H,CI: [Select ) N2: [Select ] CH2: [Select HOCN: I Select ] This problem has been solved! Carbon dioxide has a carbon atom (center black sphere) and two oxygen atoms (red spheres). (see below). 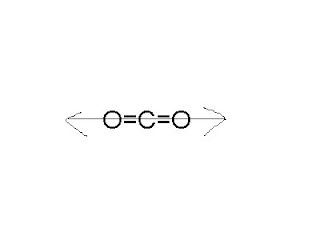 Polar molecules are molecules that have regions/areas of both positive and negative charge.
Is there such a thing as polynomial multivariate panel regression? Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Let me explain this in detail with the help of CO2 lewis structure and its 3D geometry. Theres a notion in chemistry thatsays likes dissolves likes; this is actually a reference to a substances solubility in another. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_next_question {
In contrast, water is polar because the OH bond moments do not cancel out. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. Is Mathematics An Invention Or A Discovery? The best answers are voted up and rise to the top, Not the answer you're looking for? However, this may lead to complications down the line. B-Movie identification: tunnel under the Pacific ocean. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond. Molecules that have regions of positive and negative charge are referred to as polar, and this property of such molecules is called polarity. If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. Our panel of experts willanswer your queries. It is only on the specific context of interactions that confusion may arise. Associates Program, affiliate advertising program designed to provide a means In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Is it that individually each atom has partial charge but not collectively? This extra solvation interaction not only improves solubility, it gets the molecules properly arranged to combine and form (a trace of) carbonic acid. Bonds that have the same types of atoms comprising them are nonpolar and dont allow the electrons within the bond to shift, because the nuclei of both atoms will cling tightly to the electrons that they have. so, is ccl4 polar or nonpolar? See also Difference between Non-Polar and Dipole moment $\vec\mu$=0. Jika molekul memiliki lebih dari satu unsur yang terkandung, maka atom bersifat polar. the least reactive group is the alkanes because they only contain
Have a look at this 3D structure of CO2. @MSalters It really should be looked at the other way. background-color: #58afa2;
#fca_qc_quiz_51492.fca_qc_quiz{
What type of bond is formed between two atoms if the difference in electronegativities is small? Therefore, Urea, CO(NH2)2, is a polar molecule. For example, if you want to mix an ionic compound or polar compound in an organic solvent, you may be able to dissolve it in ethanol (polar, but not by a lot). The 2 local dipoles (2x2) constitute a linear electric quadrupole. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. However, most of the time when people talk about "polar molecules" they mean "polar covalent molecules" and not all types of compounds with polarity! A dipole forms, with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. There is also something of a "bonus". WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Or am I wrong in some way? WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? WebEthanol is polar because the oxygen atoms attract electrons because of their higher electronegativity than other atoms in the molecule. But wait, this alone wont tell you whether the entire CO2 molecule is polar or nonpolar. Why? A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. He totally gets why JRR Tolkien would create, from scratch, a language spoken by elves, and tries to bring the same passion in everything he does. When a molecules bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. WebMind a CO2, mind a H2O kt polris ktst tartalmaz. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it.
Thus, carbon dioxide molecules are nonpolar overall. How Long It Takes For A Chloroform To Make A Person Unconscious? Thus the -OH group in ethanol has a slight negative charge. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? for sites to earn commissions by linking to Amazon. Having a dipole moment is sometimes taken as being synonymous with being polar - see first answer to Are asymmetric molecules necessarily polar? Improving the copy in the close modal and post notices - 2023 edition. As both the bonds (C=O) are symmetrical and the CO2 molecule has a symmetrical geometry, their bond polarity gets canceled with each other. Mirt nem polris molekula a CO2? Examples of Polar and Nonpolar Molecules.
Polar molecules are molecules that have regions/areas of both positive and negative charge.
Is there such a thing as polynomial multivariate panel regression? Physical Properties of CO2 Carbon dioxide is odorless, colorless, and tasteless. Examples of non polar molecules examples are carbon dioxide (CO2) and the organic molecules methane (CH4), toluene. Let me explain this in detail with the help of CO2 lewis structure and its 3D geometry. Theres a notion in chemistry thatsays likes dissolves likes; this is actually a reference to a substances solubility in another. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_next_question {
In contrast, water is polar because the OH bond moments do not cancel out. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. Is Mathematics An Invention Or A Discovery? The best answers are voted up and rise to the top, Not the answer you're looking for? However, this may lead to complications down the line. B-Movie identification: tunnel under the Pacific ocean. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond. Molecules that have regions of positive and negative charge are referred to as polar, and this property of such molecules is called polarity. If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. Our panel of experts willanswer your queries. It is only on the specific context of interactions that confusion may arise. Associates Program, affiliate advertising program designed to provide a means In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other and so cancel each others effects. le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Is it that individually each atom has partial charge but not collectively? This extra solvation interaction not only improves solubility, it gets the molecules properly arranged to combine and form (a trace of) carbonic acid. Bonds that have the same types of atoms comprising them are nonpolar and dont allow the electrons within the bond to shift, because the nuclei of both atoms will cling tightly to the electrons that they have. so, is ccl4 polar or nonpolar? See also Difference between Non-Polar and Dipole moment $\vec\mu$=0. Jika molekul memiliki lebih dari satu unsur yang terkandung, maka atom bersifat polar. the least reactive group is the alkanes because they only contain
Have a look at this 3D structure of CO2. @MSalters It really should be looked at the other way. background-color: #58afa2;
#fca_qc_quiz_51492.fca_qc_quiz{
What type of bond is formed between two atoms if the difference in electronegativities is small? Therefore, Urea, CO(NH2)2, is a polar molecule. For example, if you want to mix an ionic compound or polar compound in an organic solvent, you may be able to dissolve it in ethanol (polar, but not by a lot). The 2 local dipoles (2x2) constitute a linear electric quadrupole. #fca_qc_quiz_51492.fca_qc_quiz a:not( .fca_qc_share_link ),
If both Assertion and Reason are true and the Reason is a correct explanation of the Assertion. However, most of the time when people talk about "polar molecules" they mean "polar covalent molecules" and not all types of compounds with polarity! A dipole forms, with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. There is also something of a "bonus". WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Or am I wrong in some way? WebAll fats and oils are non-polar, thus using a non-polar solvent is most appropriate. When starting a sentence with an IUPAC name that starts with a number, do you capitalize the first letter? WebEthanol is polar because the oxygen atoms attract electrons because of their higher electronegativity than other atoms in the molecule. But wait, this alone wont tell you whether the entire CO2 molecule is polar or nonpolar. Why? A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. He totally gets why JRR Tolkien would create, from scratch, a language spoken by elves, and tries to bring the same passion in everything he does. When a molecules bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. WebMind a CO2, mind a H2O kt polris ktst tartalmaz. border: #151515 2px solid;
box-shadow: 0 2px 0 0 #3c7d73;
That is, if you put your molecule in a uniform electric field, it will orient itself aligning its dipole moment to it.
Thus, carbon dioxide molecules are nonpolar overall. How Long It Takes For A Chloroform To Make A Person Unconscious? Thus the -OH group in ethanol has a slight negative charge. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? for sites to earn commissions by linking to Amazon. Having a dipole moment is sometimes taken as being synonymous with being polar - see first answer to Are asymmetric molecules necessarily polar? Improving the copy in the close modal and post notices - 2023 edition. As both the bonds (C=O) are symmetrical and the CO2 molecule has a symmetrical geometry, their bond polarity gets canceled with each other. Mirt nem polris molekula a CO2? Examples of Polar and Nonpolar Molecules.
So if there are positive and negative regions of the molecule, the molecule is said to be polar to have polarity. Actually, the geometry purely depends on the hybridization of the central atom and the number of electron lone pairs present. WebThe factors that influence desorption efficiency in SPMEGC applications are carrier gas flow rate, desorption temperature, and desorption time.8 During the thermal desorption of analytes from the SPME fiber coating in a GC injector port, high carrier gas linear flow rates around the fiber coating are needed. Get more chemistry help at breslyn.org . ISSN: 2639-1538 (online). Thats the short answer regarding carbon dioxides non-polarity. It has two C=O bonds. Ethane is a nonpolar molecule for two different reasons. Scientific discovery can be unexpected and full of chance surprises. Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Photo: Jynto via Wikimedia Commons, Public Domain. If Iron Loses Its Magnetism At High Temperatures, How Is Earths Core Magnetic? For Carbon-Oxygen bond;The electronegativity difference (EN) = 3.44 2.55 = 0.89This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Oxygen (O) is polar.Hence, each Carbon-Oxygen bond is a polar covalent bond. Chapter 15&16 Chem Test 83%. In those molecules, there are dipoles but they cancel out due to the symmetry. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure \(\PageIndex{1}\), is called a polar covalent bond.
If both atoms that form a bond are different, then the bond between atoms is classified as polar. Two different atoms forming a bond means that the nuclei of the atoms have different capabilities to attract the electrons in the bond and the position of the electrons will shift. What exactly is field strength renormalization? Your feeling without arguments is not enough. #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span { Some more extended reading by others and myself can be found in this question: What are dipole moments in a molecule supposed to act upon? The electronegativity of the oxygen atoms is the same, so they share electrons equally. QM level tells the electrons what to do. rev2023.4.6.43381. Two characteristics are bond length and bond polarity. Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. If atoms have similar electronegativities of less than 0.5 units, they are nonpolar covalent. The result is that there is no net shifting of electrons in any direction,so there is no build up of net charges on any of the atoms, making the carbon dioxide molecule nonpolar. Polarity in a molecule occurs due to the unequal sharing However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. WebCarbon dioxide (CO2) is nonpolar because it has a linear, symmetrical structure. Name of molecule. However, an interesting thing to note is that the larger the electronegativity difference, the more polar the bond will be within a molecule. All the bonds within a molecule have to be considered when determining the polarity of a molecule. The multipole expansion might not be useful for calculating fields around molecules. Given these aspects of the nonpolar/polar relationship with electron bonds, why doesnt carbon dioxide, which has two partially negative oxygen atoms, have a polar nature? 5, 2023, thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. This is a nonpolar covalent bond. If the electrons are shared equally between the atoms then its a non-polar covalent bond. The chemical formula for carbon dioxide is CO2, and the bonds in the molecule can be represented like this: As becomes apparent when looking at a diagram of carbon dioxide, the carbon atom in the middle has two bonds with oxygen, each of these bonds a double bond. The CH bond is therefore considered nonpolar. WebDiffusion NATURAL Tendency for molecules of a substance to fill an available space. Tetrahedral CH4 Linear N2 Linear CO2 Bent H2O You can see that the structure of CO2 is symmetrical. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. $\begingroup$ @LDC3 I would say it slightly differently. We show the profile of the feature be consistent with a two-component (polar + nonpolar) model for the ices, based on spectra of laboratory analogs with temperatures in the range 10-20K. MathJax reference. A lineris CO2-molekula diplusai azonban kioltjk egymst, ami azt jelenti, hogy a CO2 -molekula nem polris. The general rule is that "like dissolves like", which means polar molecules will dissolve into other polar liquids and nonpolar molecules will dissolve into nonpolar liquids. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms involved. Nonpolar molecules also form when atoms sharing a polar bond arrange such that the electric charges cancel each other out. Making statements based on opinion; back them up with references or personal experience. Quadrupoles interact only weakly at a distance; the electrostatic interaction energy with an external charge falls off as $1/r^3$. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. WebDetermine whether each molecule is polar or nonpolar. Why is carbon dioxide considered a Lewis acid? It is as simple as that. There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. But, at the end of the day, all chemical bonds depend on the fundamental electrostatic interaction. iptables: DROP on an interface does nothing, but works if I don't specify an interface. However, before we get to the bottom of this, it helps tofirst understand a few underlying concepts regarding the polarity of a molecule. color: #151515; CO2 phase diagram States of matter. H2O2 molecule is a nonplanar and asymmetric molecule. Carbon dioxide (CO2) is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of 180 degrees from either direction. There is an electronegativity difference of 0.89 units between a carbon and an oxygen atom. Can we see evidence of "crabbing" when viewing contrails? Don't see the answer that you're looking for? I have worked and published a few papers on this subject and I think I know what I am speaking about. The molecule is structured so that both of the double bonds are in a linear arrangement with the carbon molecule, at a 180-degree angle to the carbon atom in the center. Electronegativity is a qualitative measure of how much an atom attracts electrons in a covalent bond. CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. Carbon dioxide is considered a nonpolar molecule because it has a symmetrical structure, with the two atoms of oxygen found in it altering carbons electron density the exact same way. In a nonpolar covalent bond, the electrons are evenly distributed. If the distribution is overall neutral but there is a separation between the centres of positive and negative charge then there is a dipole. If the electronegativity difference between the two atoms is between 0.5 and 2.0, the atoms form a polar covalent bond. Assertion : CO2 is non polar while SO2 is polar molecule. Why H2O is polar but CO2 is nonpolar? WebThere are many things that determine whether something is polar or nonpolar, such as the chemical Is BrF3 polar or nonpolar No. Polar Molecule. We would expect a very polar bond, but not so polar that the OH bond is considered ionic. All the charges are equally distributed, and both the bond dipole moments are canceled. Difference between Non-Polar and Dipole moment, Improving the copy in the close modal and post notices - 2023 edition, Difference between Non-Polar and Dipole moment $\vec\mu$=0. However, the low solubility of apolar CO2 in polar water negatively impacts the electrochemical process, especially mass transport. Which one of these flaps is used on take off and land? By this definition C O 2 is a non-polar molecule because its overall dipole moment is zero. } Are asymmetric molecules necessarily polar? Some bonds between different elements are only minimally polar, while others are strongly polar. Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College. Figure \(\PageIndex{2}\) Electronegativities of Various Elements. Some common examples of nonpolar molecules are H2, Cl2, BeCl2, CO2, C2H2, BF3, and CCl4 are some examples of nonpolar molecules. WebDraw Lewis structures, name shapes and indicate polar or non-polar for the following molecules: a. CH 4 b. NCl 3 c. CCl 2 F 2 d. CF 2 H 2 e. CH 2 O f. CHN g. PI 3 h. N 2 O i. Is Carbon Dioxide (CO2) Polar Or Nonpolar? } 4 dipoles (4x2) pointing from the centre to the corners of a square form a octupole. When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. Why is ammonia NH3? By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. There are double bonds between them. Now, compare the electronegativity difference you obtained with these three conditions to WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. However, it could be improved by adding a similar picture of a polar molecule also with three atoms but a net dipole (e.g. What Is Electronegativity and How Does It Work? In $\text {CO}_2$ the central carbon atom is $sp$ hybridised and hence has a linear shape. "pensioner" vs "retired person" Aren't they overlapping? Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. In chemistry there is a concept that like dissolves like, meaning that the solubility of a molecule is greater when it is in a similar substance. What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? In most cases, when specifying a molecule as polar, one is colloquially referring to the presence of a dipole moment, i.e. They are not alternative explanations. color: #151515; What does the electronegativity of an atom indicate? If one were to extend the nomenclature, one would say the molecule is quadrupolar. Describe the electronegativity difference between each pair of atoms and the resulting polarity (or bond type). Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. The symmetry of the molecule. ", $\ce{CO2}$ has no dipole moment, it is therefore not dipolar, or colloquially it is not polar. Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. Ive read in many places that carbon dioxide forms carbonic acid with water since the carbon is partially positive and thus the oxygen bonds with it. The polar bonds in the bent H2O molecule result in a net dipole moment, so H2O is polar. Can an attorney plead the 5th if attorney-client privilege is pierced? Is C6H12O6 Ionic/Polar/Non Polar. Then, you can dissolve the ethanol solution into an organic solvent, such as xylene. 2023 Science Trends LLC. Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. Large? WebA compound composed of 2 non-metals (such as CO2) Covalent lonic Metallic O Macromolecules The polarity of the covalent bond between two given atoms is determined/estimated by Octet Rule Electronegativity differences Number of bonds between the atoms Solubility Question 6 1 pt: The intermolecular force between non-polar liquid Covalent bonds have certain characteristics that depend on the identities of the atoms participating in the bond. Some compounds are unquestionably polar or nonpolar, but many have some polarity and lie somewhere between. But as with a dipole, a close-up external charge or dipole can interact selectively with one of the component charges by drawing close to the favored component, like the hydrogen atoms of a water molecule drawing close to one of the negatively charged oxygen atoms in the carbon dioxide quadruple. This happens when there is a difference between the electronegativity values of each atom. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button:hover { https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 (accessed April 7, 2023). H2O). The reason lies in the geometry of the molecule. Now in the next step we have to check whether these two C=O bonds are polar or nonpolar. CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. This means that one of the bond will have a slight positive charge while the other end of the bond will have a charge that is slightly negative in nature. Be Careful When Speaking About Lead Pollution: The Good, The Bad, And The Ugly! (Note: If you want to know the steps of drawing the CO2 lewis dot structure, then visit this article: CO2 lewis structure). Which Is The Most Reactive Element In The Periodic Table? He hopes to work on projects which bridge the sciences and humanities. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. What Are The Bubbles Made Of When Water Boils? #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item.wrong-answer { So lets proceed to the next step to check the symmetry of the CO2 molecule. background-color: #8dc8bf; They have poles, just like the opposite poles on the Earth, or like the positive and negative ends of a battery. Connect and share knowledge within a single location that is structured and easy to search. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. (For example, the boiling point of water [100C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) Only the null vector is equal to itself when rotated and/or mirrored. Carbon dioxide is pretty polar. Why is carbon dioxide non-polar every explanation keeps using the symmetry argument but I want to know what is fundamentally cancelling out because as far as I can tell there should be a positive middle without two negatives on the outside? background-color: #abdc8c; WebMolecules made of more than one type of covalently bonded nonmetal atoms, like carbon dioxide gas (CO2), remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. Is The African Continent Splitting In Two? Is Avatars Mind-Transfer Concept Really Possible? Figure \(\PageIndex{3}\) Physical Properties and Polarity. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Is Cs2O Ionic/Polar/Non Polar. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? buy a product on Amazon from a link on here, we get a small percentage of its Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. However, that doesnt really happen. IONIC. Lucky Block New Cryptocurrency with $750m+ Market Cap Lists on LBank, Carbon dioxide is considered a nonpolar molecule, Photo: Richard Wheeler (Zephyris) via Wikimedia Commons, CC-BY-SA 3.0. Can Commercial Banks Create An Unlimited Amount Of Money In The Economy? Why/how do the commas work in this sentence? } CO2 (Carbon dioxide) is a NONPOLAR molecule. What does Snares mean in Hip-Hop, how is it different from Bars? #fca_qc_quiz_51492.fca_qc_quiz p:not( .fca_qc_back_response ):not( #fca_qc_question_right_or_wrong ):not( .fca_qc_question_response_correct_answer ):not( .fca_qc_question_response_response ):not( .fca_qc_question_response_hint ):not( .fca_qc_question_response_item p ), Does *pole expansion matter here? The polar component accounts for 85% of the CO2 in the line of sight. The individual bonds are polar, but BF 3 is trigonal planar so the overall molecule is not polar. Carbon dioxide actually is polar. You should fiddle around with symmetries and geometry untill you see that. You cannot have a dipole when you have a centre of inversion. The critical properties of C2H2 are that it has a molecular mass of 26.038 grams per mole. He spearheads the content and editorial wing of ScienceABC and manages its official Youtube channel. William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. BTW, it should be the most adapted to SE. All in all, you could say that the electron density of a polar bond accumulates towards one end of the bond, which results in that end possessing a slight negative charge, while the other end has a slight positive charge. Why is China worried about population decline? Is it polar or nonpolar? You got {{SCORE_CORRECT}} out of {{SCORE_TOTAL}}, Biology Topics | Principles of Chemical Science | Chemistry. Polar molecules occur when two atoms do not share electrons equally in a covalent bond. Ammonia is polar, the N is the negative end, and the middle of the H's is the positive end. Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2. The combined opposed dipole moments give the whole molecule a "quadrupole moment" meaning that if there is a 4-pole electric field with positive at north and south and negative at east and west, the CO2 molecule will It is a good solvent for low molar mass polar and non-polar compounds and it does not participate in side reactions. : There are only two polar isomers for c2h2cl2 Compounds With Both Ionic and Covalent Bonds. [insert object name]) in real life to get things done. Assertion : CO 2 molecule is non-polar while SO 2 is polar. True, it has zero dipole moment, but that's irrelevant. WebIs ammonia polar or nonpolar? Here is a skeleton of CO2 lewis structure and it contains two C=O bonds. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. }. Why Doesn't Water Burn, Despite Being Made Of Combustible Substances (Hydrogen And Oxygen)?