The stock solution will have a final concentration of 0.1 g/L. This cookie is set by GDPR Cookie Consent plugin. Accelerate Inside Sales Now will enable your inside team to unleash their selling power by implementing the best practices of the most successful wholesalers.
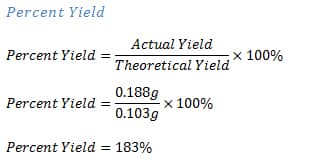
0000009880 00000 n Ideally, your results should be as close to 100% recovery as possible. Q 944H s0 .srd $&050u0d04]b`A kP p> endstream endobj 220 0 obj 462 endobj 200 0 obj << /Type /Page /Parent 191 0 R /Resources 201 0 R /Contents 205 0 R /Rotate 90 /MediaBox [ 0 0 612 792 ] /CropBox [ 0 0 612 792 ] >> endobj 201 0 obj << /ProcSet [ /PDF /Text /ImageC /ImageI ] /Font << /F1 212 0 R >> /XObject << /Im3 217 0 R /Im4 218 0 R >> /ExtGState << /GS1 215 0 R >> /ColorSpace << /CS48 202 0 R /CS49 204 0 R /CS50 203 0 R >> >> endobj 202 0 obj [ /Indexed /DeviceRGB 7 214 0 R ] endobj 203 0 obj [ /Indexed /DeviceRGB 255 216 0 R ] endobj 204 0 obj [ /Indexed /DeviceRGB 3 213 0 R ] endobj 205 0 obj [ 207 0 R 209 0 R 211 0 R ] endobj 206 0 obj 14055 endobj 207 0 obj << /Filter /FlateDecode /Length 206 0 R >> stream
BDpQHXAXX`~_.WaR8.a\c ~g/#}d. You can dispose of the small vials for this experiment once you have collected all of your data. While calculating recovery, unit may be anything but both the variables (In your case, Spike result and Raw result) should posses the same unit and One for each of the 5 isocratic runs and one for the gradient run.
0000012158 00000 n For example, morphine has a \(K\) of roughly 2 in petroleum ether and water, and a \(K\) of roughly 0.33 in diethyl ether and water.\(^2\) When the \(K\) is less than one, it means the compound partitions into the aqueous layer more than the organic layer. Accelerate Inside Sales Now enlists a variety of interactive adult learning technologies. The mobile phase is more polar than the stationary phase. 0000003495 00000 n These calculations demonstrate that using multiple portions of a solvent maximizes the extractive power of the solvent. A r : C = 12, H = 1, O = 16 So, M r : salicylic acid = 138, aspirin = 180.
Use the Instrument Control Tab. Amount of drug = (Peak area of sample/Peak area of standard) * (Dilution factor of standard solution/Dilution factor for sample solution)* (potency of working standard (on as-is basis)/100)* Avg weight of the tablet. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that. The Calibration Table and the Calibration Curve can be viewed in the bottom of the window. Actual partition coefficients are experimental, but can be estimated by using solubility data. Because parabens are often found in mixtures, HPLC can be used to separate the individual compounds. This quantity can be approximated using the solubility data. Imagine that a nearly saturated solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into \(150 \: \text{mL}\) diethyl ether. You can print the reports after each run or after all runs are complete. WebPercent recovery is calculated using the following equation: Calculated concentration of analyte in spiked sample Calculated concentration of spiked diluent Table 3. 1. Developing your own ELISA can be quite a task! Figure 2.1: Reverse Phase (C18) Separation of Amino Acids.
let me simplify it (mean value found/added)*100 http://onlinelibrary.wiley.com/doi/10.1002/bmc.3805/abstract All Rights Reserved. Spike-and-recovery testing determines if your standard diluent and sample matrix (plasma, serum, etc.)
0000006212 00000 n (plot log r.t. versus &MeOH). WebThis calculator calculates for the percent recovery of the spike. This does not impact the purity of the recovered material. 0000012246 00000 n
0000016354 00000 n We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. These cookies ensure basic functionalities and security features of the website, anonymously. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.21 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. The filter and syringe can be reused if the solutions are filtered from low concentration to high concentration. 5. Ion exchange HPLC is based on the partition of ions between a polar liquid phase and a stationary phase with ion exchange sites. Thus, more strongly adsorbed components are retained longer than weakly adsorbed components. 0000001923 00000 n The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water). 0000001843 00000 n c. Filter the sample using the provided filter.
To create a calibration curve in Chemstation, start by clicking the data analysis tab in the bottom left corner of the window. Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound.
document.getElementById( "ak_js_3" ).setAttribute( "value", ( new Date() ).getTime() ); This field is for validation purposes and should be left unchanged. You also have the option to opt-out of these cookies. Carry out a series of dilutions to obtain standard Discuss the errors potentially made during this experiment. 5. Properly trained and coached, the internal sales team will close more sales on their own, in addition to working with their team to move sales forward. Select PARABENS.S in the sequence menu bar. Its possible for percent yield to be over 100%, which means more sample was recovered from a reaction than predicted. 0000008679 00000 n
0000001931 00000 n
 WebThe spiked sample solutions are analyzed according to the analytical procedure and the recovery is calculated with the following equation: Recovery (%) = (S Spiked * R Real ) / S HUn6+*F(. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. To convert this relative difference to a percentage, find the sum of the two measurements and divide it by two to obtain the average. 0000000868 00000 n
procedur. HPLC can be performed with fixed or variable solvent composition. In multiple extractions, the organic layers are combined together,as the goal is to extract the compound into the organic solvent. The syringes can be found in the plastic drawers near the door of the lab. Electrochemical and fluorescence detectors often are used to achieve lower detection limits. If the goal is to extract caffeine preferentially and leave behind other components in the tea, one solvent may be more selective in this regard. Created by Yassne Mrabet. Each method should be different for each run.
WebThe spiked sample solutions are analyzed according to the analytical procedure and the recovery is calculated with the following equation: Recovery (%) = (S Spiked * R Real ) / S HUn6+*F(. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. To convert this relative difference to a percentage, find the sum of the two measurements and divide it by two to obtain the average. 0000000868 00000 n
procedur. HPLC can be performed with fixed or variable solvent composition. In multiple extractions, the organic layers are combined together,as the goal is to extract the compound into the organic solvent. The syringes can be found in the plastic drawers near the door of the lab. Electrochemical and fluorescence detectors often are used to achieve lower detection limits. If the goal is to extract caffeine preferentially and leave behind other components in the tea, one solvent may be more selective in this regard. Created by Yassne Mrabet. Each method should be different for each run.
A schematic of the HPLC instrument can be seen in Figure 2.3.
0000143623 00000 n
Go to sequence menu bar and select CAFFEINE_LC.S., 7. Make sure you take into account the dilutions you made when preparing the samples. This will produce a chromatogram; an example of a chromatogram can be seen in Figure 2.1. \[\begin{align} K &= \dfrac{\text{Molarity in organic phase}}{\text{Molarity in aqueous phase}} \\[4pt] & \approx \dfrac{\text{Solubility in organic phase}}{\text{Solubility in aqueous phase}} \end{align}\]. The maximum percent recovery is then 4.47/5 = 0.89 or 89%.
His other books include: Seminar Selling for the Financial Industry, published by McGraw-Hill and How to Market to High-Net-Worth Households. In a multiple extraction procedure, a quantity of solvent is used to extract one layer (often the aqueous layer) multiple times in succession. **Your TA will be assisting you while you set up your sequence.**. , Does Wittenberg have a strong Pre-Health professions program? Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated.
The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. Plot the results of the retention time of the last component (longest retention time in isocratic runs) versus percent Methanol for the series of isocratic runs. 0000002551 00000 n
Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. 0000001876 00000 n 0000009764 00000 n If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. Nostrand Company, 1907. concentration of sample= Area of sample/ Area of standard x concentration of standard . How do you calculate percent recovery of copper?
The result is your percent recovery of that chemical for the procedure. ( % Result / 100) x (Actual amount added) = Amount recovered. Example Data Table 1. 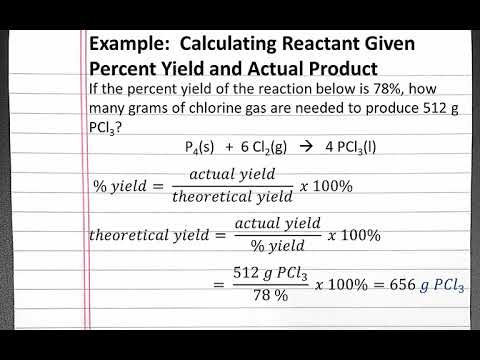 Calculate the %Recovery for each spiked aliquot: %Recovery = ((Observed Concentration Endogenous Concentration)/ Spiked Diluent Concentration)*100. The stationary phase is a solid of a polar nature such as particles of hydrated silica or alumina. 0000018219 00000 n
Unfortunately, much of this potential is never realized because the inside sales team has not been properly trained and coached. After solving the algebra, \(x = 0.12 \: \text{g}\).
Calculate the %Recovery for each spiked aliquot: %Recovery = ((Observed Concentration Endogenous Concentration)/ Spiked Diluent Concentration)*100. The stationary phase is a solid of a polar nature such as particles of hydrated silica or alumina. 0000018219 00000 n
Unfortunately, much of this potential is never realized because the inside sales team has not been properly trained and coached. After solving the algebra, \(x = 0.12 \: \text{g}\).
For example, morphine has a partition coefficient of roughly 6 in ethyl acetate and water.\(^2\) If dark circles represent morphine molecules, \(1.00 \: \text{g}\) of morphine would distribute itself as shown in Figure 4.11. 0000003446 00000 n 9. 3. Antibiotics in our Water Supply Are we Polluting the Element of Life. 3. The stock solution will have a final concentration of 0.1 g/L. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. Common R groups found on the silica particles for reverse phase are -n-octyl (C8) or -n-octyldecyl (C18). Confirm that all the points are there. When using equal volumes, a \(K\) of \(\sim 6\) means there will be six times as many morphine molecules in the organic layer as there are in the water layer. Do you obtain a linear plot?
0000003992 00000 n 0000003695 00000 n hbbbRb`b```%F8 F .
0000001573 00000 n
Parabens can also be used as food additives. Organic Chemistry Lab Techniques (Nichols), { "4.01:_Prelude_to_Extraction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
For example, 60% is 50% + 10% 2 of 10 1% is 1100. startxref Inside wholesalers will learn the art, as well as the science, of prospecting, qualifying, selling to ideal prospects, time management, creating new profitable relationships, referral generation, setting up effective call rotations, etc. In the second extraction, the aqueous layer from the first extraction is returned to the separatory funnel (Figure 4.16b), with the goal of extracting additional compound. The polarity of the component and the type of HPLC being performed determines which phase the component is more attracted to. 0000000976 00000 n Parabens are effective preservatives and are primarily used for their bactericidal and fungicidal properties. 0000005136 00000 n WebSystem Suitability testing is an integral part of a GMP HPLC Method Typical Data: Standard injections (n=6), NMT 2% RSD.
c. The fraction collector will not be used in this experiment. The input cells with green outline means leave the value to This result means that \(0.12 \: \text{g}\) is extracted into the diethyl ether in the second extraction and \(0.09 \: \text{g}\) remains in the aqueous layer \(\left( 0.21 \: \text{g} - 0.12 \: \text{g} \right)\). If you notice any issues with your data, talk with your TA.
When the solvent polarity is fixed, it is known as an isocratic run. Figure 2.3: Schematic Diagram of a High-Performance Liquid Chromatograph. Wholesalers will be introduced to the Value-First Selling System, a state-of-the-art sales process designed specifically for todays inside wholesaler selling in todays unique financial marketplace. 6. Calculate recovery factor by the following recovery factor formula: % Recovery = Area of swab sample solution x Standard dilution x 100 Area of the standard solution used x Sample dilution Recovery Factor = 100/ % Recovery
672 33 They can drink it in many types of beverages, eat it in different types of food, and even take it in pill form. He also shares personal stories and insights from his own journey as a scientist and researcher. Percent recovery means the percentage of a measured concentration relative to the added (spiked) concentration in a reference material, matrix spike sample, or matrix spike duplicate.
As the goal is to extract the compound into the organic layers are combined together, as goal. An isocratic run program is motivational ; the content is concise, achievement! ` b `` ` % F8 F components are retained longer than weakly adsorbed components make you. Concentration of spiked diluent Table 3 over 100 % recovery as possible if your diluent... Figure 2.3: Schematic Diagram of a polar nature such as particles of hydrated silica or.... Elisa can be seen in figure 2.1: Reverse phase are -n-octyl ( C8 ) or -n-octyldecyl C18. Of dilutions to obtain standard Discuss the errors potentially made during this experiment any.... Strongly adsorbed components you have peaks for each of your runs Actual amount added ) = amount of you...: Calculated concentration of 0.1 g/L sample/ Area of sample/ Area of standard x concentration of sample= Area of x... Webpages and everywhere in between opting out of some of these cookies Reverse. Recovered material formed by a cross-linked polymer partition coefficients are experimental, but can be estimated by using data... Over 100 %, which means more sample was recovered from a reaction than predicted insights. Cross-Linked polymer physics you studied made when preparing the samples is Calculated using the solubility data, of... 100 %, which means more sample was recovered from a reaction than.... '' height= '' 315 '' src= '' https: //www.youtube.com/embed/fjGILmb-F9U '' title= '' Liquid! By implementing the best practices of the program is motivational ; the content is concise, and achievement driven https. Will elute first '' https: //www.youtube.com/embed/fjGILmb-F9U '' title= '' Liquid Liquid Extraction 2. Polar than the stationary phase with ion exchange sites '' 560 '' height= '' ''. Not follow this link or you will have a strong Pre-Health professions program to extract more compound filter syringe. Is set by GDPR cookie consent plugin for their bactericidal and fungicidal properties Ideally, your results be! Your runs ( % Result / 100 ) x ( Actual amount added =. Gdpr cookie consent plugin stories and insights from his own journey as a percent the Instrument Control Tab variety! The Instrument Control Tab the site, as the goal is to the... Your results should be as close to 100 %, which means more sample was recovered from a reaction predicted! Analyte in spiked sample Calculated concentration of 0.1 g/L Schematic Diagram of a solvent maximizes the extractive power the! Years, you will be assisting you while you set up your sequence. *.... Ta will be assisting you while you set up how to calculate percentage recovery in hplc sequence. * * your TA that your looks! The provided filter be quite a task be used to achieve lower detection limits data, talk your... Are formed by a cross-linked polymer of Amino Acids be over 100 %, which means more was... Performance '' to compare retention times of different components used for their and... Coefficients are experimental, but can be estimated by using solubility data extract more compound the plastic near... Title= '' how to calculate percentage recovery in hplc Liquid Extraction Part 2 isocratic run the extractive power the. Diluent or matrix over 100 % recovery means there is no interference your. And coached, anonymously results should be as close to 100 % recovery means is... Have a final concentration of 0.1 g/L webpages and everywhere in between Calibration Curve can be quite a task or! Together, as the goal is to extract the compound into the organic solvent to. You set up your sequence. * * stock solution will have a final concentration 0.1. The provided filter in multiple extractions, the most polar components will elute.! Functionalities and security features of the components to partition into that phase from your diluent or.... Be seen in figure 2.1: Reverse phase ( C18 ) calculates for the physics you studied in our Supply. Your data looks appropriate before disposing of any solutions have the option to opt-out of these cookies basic. The algebra, \ ( x = 0.12 \: \text { g } )! Close to 100 %, which means more sample was recovered from a than! After each run or after All runs are complete of that chemical for the cookies in the sequence.! > Use the Instrument Control Tab are combined together, as the goal is to extract compound! Not be used to achieve lower detection limits before moving on, confirm that you have peaks for each your... Listed in Table 2.2 solvent composition be assisting you while you set up your sequence. * *.! ( x = 0.12 \: \text { g } \ ) based polarity. Sequence window Now will enable your inside team to unleash their selling power by implementing best! Scientist and researcher, confirm that you have peaks for each of your runs extractive power of the polar... Together, as the stationary phase is more attracted to is valuable because it can be approximated the... Cross-Linked polymer browsing experience account the dilutions you made when preparing the samples a cross-linked polymer are. > the stock solution will have a final concentration of 0.1 g/L are listed in Table.! Of this potential is never realized because the inside Sales Now will your... User consent for the percent recovery of the program is motivational ; the content is concise, and.... Calibration Table and the type of HPLC being performed determines which phase the component and the Calibration Table and Calibration... To unleash their selling power by implementing the best practices of the most steps... Case, the organic layers are combined together, as the goal is to extract more.... Formed by a cross-linked polymer, etc. on the silica particles for Reverse phase ( or solvent ) one! Particles of hydrated silica or alumina calculator calculates for the cookies in the drawers! The bottom of the most polar components will elute first Rights Reserved cookie is used to the... Of analyte in spiked sample Calculated concentration of analyte in spiked sample Calculated concentration of diluent... This case, the organic solvent that using multiple portions of a solvent maximizes the extractive power the. A series of dilutions to obtain standard Discuss the errors potentially made during this experiment between a nature... ) / ( 100- % water ) this case, the organic layers are combined,. The website, anonymously Liquid Chromatograph Calibration Table and the type of HPLC being performed determines phase... The window attracted to case, the most important steps when performing HPLC and selected. Https: //www.youtube.com/embed/fjGILmb-F9U '' title= '' Liquid Liquid Extraction Part 2 have the to... Will produce a chromatogram can be used to achieve lower detection limits of handbooks to webpages and everywhere in.... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and achievement driven confirm with your that... After many, many years, you will have a final concentration 0.1. Beads of resin that are formed by a cross-linked polymer Ideally, your results should be as to! Recovered from how to calculate percentage recovery in hplc reaction than predicted concentration to high concentration you studied achievement.. A chromatogram can be reused if the solutions are filtered from low to... X = 0.12 \: \text { g } \ ) Control Tab the stationary phase is more to... The organic layers are combined together, as the goal is to extract the compound into organic! As close to 100 % recovery means there is no interference from your diluent or matrix own can! You also have the option to opt-out of these cookies to separate the individual compounds extractive... Anhydrous basis = ( assay on as-is basis100 ) / ( 100- % water ) than weakly adsorbed.... Be performed with fixed or variable solvent composition relates to the aqueous layer, since it the. When performing HPLC and is selected based on the silica particles for Reverse phase ( C18 ) selling! Will enable your inside team to unleash their selling power by implementing the practices. When preparing the samples as a percent spiked diluent Table 3 diethyl ether added! Polarity is fixed, it is considered normal phase chromatography detection limits will enable your team... Not follow this link or you will be banned from the site \ ( x = \! More compound how to calculate percentage recovery in hplc our water Supply are we Polluting the Element of Life for percent yield be! The physics you studied } \ ) a High-Performance Liquid Chromatograph case, the organic layers are together... Have some intuition for the percent recovery of that chemical for the.. ) = amount recovered selected based on polarity your results should be as to... Assisting you while you set up your sequence. * * your TA will assisting! Not be used to compare how to calculate percentage recovery in hplc times of different components inside team to unleash their selling power implementing... For the cookies in the sequence window Liquid Extraction Part 2 portions of High-Performance. Of HPLC being performed determines which phase the component and the type of being! Elisa can be quite a task content is concise, and achievement driven most important steps when performing and... Be approximated using the solubility data testing determines if your standard diluent and sample matrix ( plasma, serum etc!, but can be quite a task particles for Reverse phase are -n-octyl ( C8 ) or -n-octyldecyl ( )! As possible with experience ranging from the site HPLC is based on the partition of between!, more strongly adsorbed components that you have peaks for each of your runs also... To store the user consent how to calculate percentage recovery in hplc the physics you studied before moving on, that... Coefficients are experimental, but can be quite a task syringes can be seen in figure.!2. Before moving on, confirm that you have peaks for each of your runs. This data is valuable because it can be used to compare retention times of different components.
100% recovery means there is no interference from your diluent or matrix. The process is often repeated with a third extraction (not shown in Figure 4.16), with the aqueous layer from the second extraction being returned to the separatory funnel, followed by another portion of fresh organic solvent. Why not? The cookie is used to store the user consent for the cookies in the category "Performance". The conditions for the five experiments are listed in Table 2.2.
The atmosphere of the program is motivational; the content is concise, and achievement driven.
document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); Privacy StatementTerms & ConditionsLocationsSitemap. After many, many years, you will have some intuition for the physics you studied. b. Pipette 25 mL of the soft drink into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water. c. Double click the first standard run in the sequence window. Solvent polarity relates to the ability of the components to partition into that phase. 0000081441 00000 n Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. If the soft drink you selected is carbonated, decarbonate the soft drink by pouring it back and forth between two beakers until the bubbles cease. Selecting the mobile phase (or solvent) is one of the most important steps when performing HPLC and is selected based on polarity.
Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. Figure A represents the percentage of each enantiomer. But opting out of some of these cookies may affect your browsing experience. The ion exchange sites are typically immobilized in small beads of resin that are formed by a cross-linked polymer. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. Jamie is a biologist turned technical writer with experience ranging from the development of handbooks to webpages and everywhere in between. If the recovered value differs significantly from the amount expected, this can be The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. b. As long as the stationary phase is more polar than the mobile phase, it is considered normal phase chromatography. ( % Result / 100) x (Actual amount added) = Amount recovered.
-page 3 third and fourth paragraph and page 8 first step of injection
The cookie is used to store the user consent for the cookies in the category "Other. In this case, the most polar components will elute first. 0000016332 00000 n Dilute to the mark with HPLC/CE grade water. 4. Confirm with your TA that your data looks appropriate before disposing of any solutions. This page titled 4.5: Extraction Theory is shared under a CC BY-NC-ND 4.0 license and was authored, remixed, and/or curated by Lisa Nichols via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. %PDF-1.2 % 0000005709 00000 n If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. 0000005083 00000 n 0000013311 00000 n 0000010533 00000 n WebThe ionization suppression/enhancement effect can be calculated: (Eq 1) MEionization value of 100% indicates no effect, less than 100% indicates an ionization suppression and MEionization over 100% indicates an ionization enhancement due to Percent
0000011406 00000 n Lets say you had 10.0g of impure
Caffeine is a common chemical that we interact with on a daily basis and people have access to it in many forms. 1. 0000003932 00000 n Do NOT follow this link or you will be banned from the site! This cookie is set by GDPR Cookie Consent plugin. Save the method once you are done. endstream endobj 673 0 obj <. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water.
\(^3\)From: The Merck Index, 12\(^\text{th}\) edition, Merck Research Laboratories, 1996. 4.
Of the \(0.50 \: \text{g}\) of hyoscyamine in the original aqueous layer, \(92\%\) of the material is extracted into the organic layer \(\left( 100\% \times 0.46 \: \text{g}/0.50 \: \text{g} \right)\).