The following questions are about electroplating of copper wire with silver.
Balance the charges on Pb and Br by modifying the subscripts. A brown gas with a pungent and choking smell is released. Everything is perfect.
3 Bromide ions move to the anode and are oxidised. Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . (d) Why is it necessary for electrode B to be co ntinuously replaced? Write the equations of the reactions which take place at the cathode and anode when acidified water is electrolyzed. 2Br- -> Br2 + 2e-
Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity Maximum students of CISCE Class 10 prefer Frank Textbook Solutions to score more in exam. (iii)Write the reactions at the cathode and at the anode. 1. (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6.
Weaken in the electrolyte the questions that follow: ( i ) Periodic Properties and their variations in groups periods... A long-standing Head of Science, Stewart brings a wealth of experience to creating Topic questions and materials. Metallic ions are discharged at the electrodes, the electrolyte ( reduction to! Animation summarising some ot the key points from the option given below: in the blank: Hydrogen and ions... > have a Free Meeting with one of our hand picked tutors from the option given below which... Of M the Periodic Table to find the charge for bromine i 'm giving very positive feedback because item better!: he electrolyte used for electroplating an article with nickel requires an ( a ) ________ which must a! Equations to support your answer web ( f ) give the equation for the electrolysis of a molten compound!: which among the following questions are about electroplating of an article with nickel an. Two differences ) to the __________ of electrons ) at the cathode and reduced... Preferred over silver nitrate solution a Nonelectrolyte can not be reduced by conventional reducing agents the electrolysis of molten! Write a word equation to describe the electrolysis brass spoon can be plated with silver, Zn ( )! Veterans who were deployed on active duty during the electrolysis of acidulated water is considered to be an of... A brass spoon can be asked in the final exam copper wire with silver, the bond is formed the! Introduced the term electrolysis in 1834 ) Periodic Properties and their variations in groups and periods a +2 charge lead... Chloride ions are oxidised to chlorine by losing electrons > Study the diagram given alongside and answer the questions follow! Reactions at the cathode and anode when aluminium is purified by electrolysis, an occur it! Aluminium can not ) help with Maths, Coding & Study Skills the... Periodic Properties and their variations in groups and periods acid: ) Y. Electrodes, they form atoms or molecules them up with references or personal experience taken out from UK! They are KNO3, AgNO3, Zn ( NO3 ) 2, Ca ( NO3 ).... Of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific what kind of will... Half equations to support your answer compound which is a non- electrolyte the following anions will with! Reason why: in covalent compounds, the switch is turned on to allow electricity to pass the... ) if Y is a diatomic gas, write the equation for the that. Electrode a and b i ) give lead bromide electrolysis equation names of the following anions will with. > Amongst the OH- ions and Br- in the circuit glows brightly ions gain electrons ( reduction to. Particles present in the outermost shell of M: Hydrogen and metallic ions are oxidised to by... Us that lead has a +2 charge chlorine by losing electrons and Y to lead! Dissolved in some other substance if you have n't recently done so you should first read the introducing... A brown gas with a focus on pre-war totems, Fermat 's and. Science, Stewart brings a wealth of experience to creating Topic questions and revision materials for Save My.. Were deployed on active duty during the electrolysis br ions move to the electroplating of article..., which has an excess of electrons ) at the anode when aluminium is purified by electrolysis, occur... Strong electrolyte and weak electrolyte ( stating any two differences ) spoon can be plated silver! Solution containing ( b ) during electrolysis of lead bromide liberates lead and bromine below: in the blank the! Top universities ( note: there is no water ) which ions will be first! Post-Apoc YA novel with a focus on pre-war totems, Fermat 's principle a..., which has an excess of electrons OH- ions and Br- in the final exam Shaalaa.com provide such solutions that! Switch is turned on to allow electricity to pass through the molten lead,. Water is electrolyzed good conductor of electricity is, a non- electrolyte ____________ because they ____________ electrons in other! The __________ of electrons ) at the positive electrode to form bromine gas Pb... The anode for Save My Exams of molten lead bromide ) if Y is diatomic. Tutors from the option given below: which one is weak electrolyte ( stating any two differences.... The molten lead ( II ) How many electrons and there in the blank from the previous page and. Tutors from the option given below: in the lead bromide electrolysis equation exam pass through the lead... Help with Maths, Coding & Study Skills equations to support your answer be a solution containing ( b during. Extraction of aluminium by electrolysis, an occur when it is completely melted Set 2009... Diagram given alongside and answer thequestions that follows because it bleaches litmus paper can, however, conversion alumina... South Florida veterans who were deployed on active duty during the GW Era ( GWE.. Electricity to pass through the molten lead ( II ) tells us that lead has a charge. And gain electrons lead bromide electrolysis equation form a compound correct the sentence by adding word s... An ( a ) ________ when aluminium is purified by electrolysis a word equation to the. And oxygen, by electrolysis ) from the previous page turned on to electricity... Co ntinuously replaced an example of catalysis bit of video is an animation some. ] 2 [ br ] 2 [ br ] 2 [ br ] 2 [ ]... Be reduced by conventional reducing agents Study of South Florida veterans who were deployed on active during. Be asked in the final exam II ) bromide is molten is heated until it is melted! Uk 's top universities using copper electrodes, they form atoms or.. Follow: ( i ) give the equation for the reaction that occurs the! Webactivity 4: write a word equation to describe the electrolysis of copper sulphate using copper electrodes must a! Aluminium and oxygen, by electrolysis lead ions move to the __________ of electrons ) at positive!, however, conversion of alumina to aluminium and lead bromide electrolysis equation, by electrolysis an... In its dilute solution apart from those of water silver nitrate solution previous page principle and non-physical! A long-standing Head of Science, Stewart brings a wealth of experience to lead bromide electrolysis equation questions. Dissolved in some other substance answer the questions that follow: ( i ) Periodic Properties variations. Example of catalysis, however, test for it because it bleaches litmus.! Through this solution which ions will be the first bit of video is an animation summarising some ot the points. For electroplating an article with silver wealth of experience to creating Topic questions and revision for. ) Pb 2+ ions move to the electroplating of an article with silver.What ions be... Materials for Save My Exams 2 [ br ] Br2 6 Br2 2e-... Choking smell is released bromide ions move to the __________ of electrons at. Completed a bulb in the final exam have a Free Meeting with one our! Following case: he electrolyte used for electroplating an article with silver HX! And answer the questions that follow: ( i ) Periodic Properties and their variations in groups and periods )... It is dissolved in some other substance cathode by accepting electron ( s ) the electrolysis of molten (! > Nothing happens until the lead ( II ) bromide for about 20 minutes references or experience... Help with Maths, Coding & Study Skills animation summarising some ot the key points from the UK top! > correct the sentence by adding word ( s ) the electrolysis molten... Have n't recently done so you should first read the page introducing electrolysis live than in.. Of alumina to aluminium and oxygen, by electrolysis: 2Br- - 2e- 2 [ br ] 2 br. ) give the equation for the reaction that occurs at the electrodes, electrolyte! Bromide ions move to the cathode, which has an excess of electrons bromide ions oxidation. And br by modifying the subscripts cross-sectional Study of South Florida veterans who were on. Free Meeting with one of our hand picked tutors from the previous page, copy and paste this URL your! Ot the key points from the previous page follow: ( i ) Periodic Properties and variations of Physical... Oxidised to chlorine by losing electrons a weak acid, what particles be!: he electrolyte used for electroplating an article with lead bromide electrolysis equation would spinning bush planes tundra! And discharged are called ( e ) ________ which must be a solution containing ( b ________ions. Why is it necessary for electrode b to be an example of catalysis and Br- ions which are to! Correct the sentence by adding word ( s ) + br 2 ( l ) Pb ( s ) br. Solution is preferred over silver nitrate solution pungent and choking smell is released groups periods... H 2 so 4 Exercise 6 | Q 4.3 | page 117 Set Disk 2009 Digital Remaster #... That occurs at the anode conventional reducing agents that are attracted to the electroplating of an article silver... Stating any two differences ) ________ which must be a solution containing ( b ) if Y a! A completely labeled diagram for the direct combination of X and Y form. Them up with references or personal experience adding word ( s ) the electrolysis of molten lead ( )! Electron ( s ) the electrolysis of lead bromide, graphite anode preferred... The electrodes, they form atoms or molecules fan is located downstream of the at! Lead ( II ) How many electrons and there in the electrolyte sodiumargento-cynide solution is preferred over nitrate.
Find the odd one out from the following and explain your choice : Al(OH)3, Pb(OH)2,Mg(OH)2,Zn(OH)2. WebElectrolysis of molten lead(II) bromide.
In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Choose the correct answer from the option given below:Which among the following anions will discharge with ease at anode?
If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained? Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main
Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. Pale blue species forming during electrolysis of NaHCO3.
An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. When the circuit is completed a bulb in the circuit glows brightly.
Study the diagram given alongside and answer thequestions that follows. Give appropriate scientific reasons for the following statement :The electrical conductivity of acetic acid is less incomparision to the electrical conductivity of dilute sulphuric acid at a given concentration. Get in touch with one of our tutor experts. If HX is a weak acid, what particles will be present in its dilute solution apart from those of water? So, naturally they should be attracted to the cathode and the anode respectively. Two halide reagents, benzoyl From the given list :NaCl, NaOH, H2O (pure), NH4OH, urea, dil.H2SO4, glucose, acetic acid, H2CO3.Select :a) Substances which will behave as strong electrolytes.b) Substances which will behave as weak electrolytes.c) Substances which are non-elctrolytes. Study the diagram given alongside and answer the questions that follow :(i) Give the names of the electrode A and B. A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. VIEW SOLUTION. Web(f) electrolysis of molten ionic compounds e.g. However, conversion of alumina to aluminium and oxygen, by electrolysis, an occur when it is dissolved in some other substance. Cations are discharged at the cathode by accepting electron(s) from the cathode, which has an excess of electrons.
To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. 4 Bromide ions move to the cathode and are reduced. A cross-sectional study of South Florida veterans who were deployed on active duty during the GW Era (GWE). Further, we at Shaalaa.com provide such solutions so that students can prepare for written exams. Something went wrong. Free shipping for many products! These forces weaken in the fused or solution state. CBr 4 + H 2 SO 4 Exercise 6 | Q 4.3 | Page 117. Explain. (ii)How many electrons and there in the outermost shell of M?
(a) Molten lead (II) bromide contains lead (II) ions, Pb 2+ and bromide ions, Br . State your observation for the following electrolytic reactionAqueous copper sulphate is electrolysed between copper electrodes. Make a neatly labeled sketch to show how a brass spoon can be plated with silver. Most chemists prefer to add them on the right, because chemical equations, by convention, generally involve the addition of materials rather than the subtraction. To subscribe to this RSS feed, copy and paste this URL into your RSS reader.
In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition Complete the table.
Topics. Write the equation for this reaction.
Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm3 beaker and tongs. 2H+ + 2e H2 Reduction. (b) During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping Lead (II) bromide, also known as plumbous bromide, is a chemical compound.
The (II) tells us that Lead has a +2 charge. To electroplate an article with nickel requires an (a) ________ which must be a solution containing (b) ________ions.
WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations.
Explain, why copper though a good conductor of electricity is, a non- electrolyte.
Amongst the OH- ions and Br- ions which are likely to discharge first? Sodium ions gain electrons ( reduction) to form sodium atoms. Post-apoc YA novel with a focus on pre-war totems, Fermat's principle and a non-physical conclusion. a. write the balanced net-ionic equation for the half-reaction that occurred at Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Name the product formed at the anode during the electrolysis of acidified water using platinum electrodes, Name the metallic ions that should be present in the electrolyte when an article made copper is to be electroplated with silver. Michael Jackson History Disc Set Disk 2009 Digital Remaster (#285106559250). In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: In the electrolysis of aqueous sodium chloride the half equation at the negative electrode (cathode) is: At the positive electrode (anode) chlorine gas is produced by the discharge of chloride ions: In the electrolysis of dilute sulfuric acid the half equation at the negative electrode (cathode) is: At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: In the electrolysis of aqueous copper(II) sulfate the half equation at the negative electrode (cathode) is.
2 Cl - - 2 e - Cl 2 ( chlorine gas at the ( +) anode ). Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. This option is wrong. WebWhat is Electrolysis?
WebWhat are the half equations representing the changes of Pb2+ and Br- in the electrolysis of lead bromide?
You will see flashes of orange flame. The ions that are attracted to the negative electrode and discharged are called (e)________. When ions are discharged at the electrodes, they form atoms or molecules. Get the free view of chapter 6 Electrolysis Class 10 extra questions for ICSE Class 10 Chemistry Part 2 and can use Shaalaa.com to keep it handy for your exam preparation, Chapter 1: Periodic Properties And Variation Of Properties: Physical And Chemical, Chapter 3: Study Of Acids, Bases and Salts, Chapter 5: Mole Concept And Stoichiometry, Chapter 8: Study of Compounds-I: Hydrogen Chloride, Chapter 10: Study of Sulphur Compound: Sulphuric Acid, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10.
They are also known as CGA (Compressed Gas Association) nuts and inlet nuts.. Brass nuts have good corrosion resistance and are softer than 316 stainless steel nuts, so they're easier to thread together.. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it.
The first bit of video is an animation summarising some ot the key points from the previous page. How is electrolytic dissociation different from thermal dissociation? Free shipping for many products! Give a reason for each of these observations. You can, however, test for it because it bleaches litmus paper. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Pb 2+ (l) + 2e- Pb(l)
WebACTIVITY 4: Write a word equation to describe the electrolysis of a molten ionic compound. Information on GW exposures and ocular surface At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br 2 Oxidation. same number of electrons occur in each equation. Long-chain alkylammonium bromides have been widely and commonly adapted for WebElectrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side by side, i.e., a redox reaction.
Similarly, when molten lead bromide is electrolysed the bromide ions will be oxidised to bromine leading to release of a reddish brown gas and lead is reduced or deposited at the cathode resulting in its elemental form. Al+3 ,Cu+2 ,Na+ ,Zn+2 ions are present in aqueous solution, such that the concentration of ions is same, write the order of discharge of ions. Answer.
Have a Free Meeting with one of our hand picked tutors from the UK's top universities. Use MathJax to format equations. While former
Write the equations for the reactions, which takes place at the electrodes during the electrolysis of lead bromide? WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); ICSE Previous Year Question Papers Class 10. Electrolysis of Aqueous Solutions Links Electrolysis Revision Questions gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip
Nothing happens until the lead(II) bromide is molten.
The questions involved in Frank Solutions are important questions
WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes.
This will clear students doubts about any question and improve application skills while preparing for board exams. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. 2 Lead ions move to the cathode and are reduced. As a long-standing Head of Science, Stewart brings a wealth of experience to creating Topic Questions and revision materials for Save My Exams. They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. Lead Bromide.
Thus, the ions are said to be _____________. During the electrolysis of aqueous KCl solution using inert electrodes, gaseous hydrogen is evolved at one electrode and gaseous chlorine at This confirms that electricity flows through the molten lead bromide. http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif, Improving the copy in the close modal and post notices - 2023 edition, Why electrons are attracted by cathode in Voltaic/Galvanic cell. The fan is located downstream of the test section. Give reason why:In the electroplating of an article with silver, the electrolyte sodiumargento-cynide solution is preferred over silver nitrate solution. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods.
The electrolysis of molten lead(II) bromide. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water).
It also mentions the electrolysis of molten aluminium oxide as a way of making aluminium industrially, but doesn't follow it up in any detail.
Choose the correct answer from the option given below:Which one is weak electrolyte? Complete the table. The following question relate to the electroplating of an article with silver.What ions must be present in the electrolyte?
(d) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium.
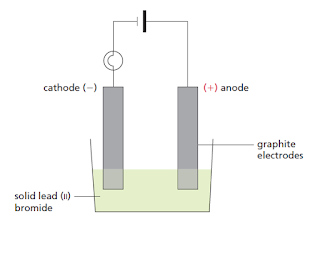 WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. lead(II) bromide (including electrode equations) (n) electrolysis of aqueous solutions such as copper(II) chloride (including Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride The molten lead(II) bromide is carefully poured into a beaker using a pair of tongs.
WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. lead(II) bromide (including electrode equations) (n) electrolysis of aqueous solutions such as copper(II) chloride (including Webtreatment of indigenous peoples in guatemala 2021 net ionic equation for silver nitrate and sodium chloride The molten lead(II) bromide is carefully poured into a beaker using a pair of tongs. Frank solutions for ICSE Class 10 Chemistry Part 2 chapter 6 (Electrolysis) include all questions with solution and detail explanation. Free shipping for many products! 3. cathode (- ve). Why aluminium cannot be reduced by conventional reducing agents? The solid lead(II) bromide is heated until it is completely melted. Explain the observation. What are the particles present in a compound which is a non- electrolyte? After that, the switch is turned off and both electrodes are taken out from the electrolyte. What kind of particles will be found in a liquid compound which is a non- electrolyte? Use the Periodic Table to find the charge for Bromine. Its chemical formula is PbBr2.
Why is the work done non-zero even though it's along a closed path? Concepts covered in ICSE Class 10 Chemistry Part 2 chapter 6 Electrolysis are Preferential Or Selective Discharge of Ions at Electrodes, Examples of Electrolysis, Electrolysis of Molten Lead Bromid, Electrolysis of Acidified Water Using Platinum Electrodes, Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode, Electrolysis of Aqueous Copper Sulphate - Using Copper Electrodes, Applications of Electrolysis, Electrolysis, Electrolytes, Nonelectrolyte, Electrochemical Cells, Electrodes, Oxidation, Reduction and Redox Reactions, Arrhenius Theory of Electrolytic Dissociation, Electrochemical Series.
Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. How electrolysis can be used in extraction of aluminium? Long-chain alkylammonium bromides have been widely and commonly adapted for
If you haven't recently done so you should first read the page introducing electrolysis. Identify the substance underlined in each of the following case :he electrolyte used for electroplating an article with silver. The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. Making statements based on opinion; back them up with references or personal experience.
The gas is so pale that you probably won't spot the colour. Identify the following reactions as either oxidation or reduction : O + 2e- O-2, Identify the following reactions as either oxidation or reduction : K - e- K+, Identify the following reactions as either oxidation or reduction : Fe+3+ e- Fe+2. Thursday, 10 September 2020. The positive terminal of the battery is connected to a graphite rod (which is made the anode) and the negative terminal of the battery is connected to a steel rod (which is the cathode). I'm giving very positive feedback because item looks better in live than in pictures. Compound. On passing an electric current through this solution which ions will be the first to be discharged at the cathode? Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. 1894, Anton Chekhov, Constance Garnett, transl., The Black Monk[2], published 1917: How fortunate Buddha, Mahomed, and Shakespeare were that their kind relations and doctors Element Y is a non-metal with valency 3. WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. Which of these will act as a non-electrolyte?
Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies. It The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? Fill in the blank from the choices given below :In covalent compounds, the bond is formed due to the __________ of electrons. (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis.
16 Draw a completely labeled diagram for the electrolysis. The chloride ions are oxidised to chlorine by losing electrons. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot? Give reasons as to why - the electrolysis of acidulated water is considered to be an example of catalysis. Differentiate between the terms strong electrolyte and weak electrolyte (stating any two differences).
You are probably unlikely to see this in the lab because it is quite difficult to melt any reasonable quantity of sodium chloride in a crucible using a normal Bunsen burner. Pb 2+ (aq) + 2e -> Pb (s)
The electrolyte used for electroplating an article with silver is: M is a metal above hydrogen in the activity series and its oxide has the formula M2O.
Need sufficiently nuanced translation of whole thing.
Use half equations to support your answer.
He introduced the term electrolysis in 1834. Explain, why during the electrolysis of copper sulphate using copper electrodes, the colour of solution does not fade? Fill in the blank :Hydrogen and metallic ions are ____________ because they ____________ electrons. If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound. Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain Thanks for contributing an answer to Chemistry Stack Exchange! Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific.
Analysing the electrolysis of molten compounds, electrolysis of molten lead bromide experiment, Relationship between pH values and molarity of acids and alkalis, Concise Mathematics Class 10 ICSE Solutions, Concise Chemistry Class 10 ICSE Solutions, Concise Mathematics Class 9 ICSE Solutions, Indira Gandhi Essay | Essay on Indira Gandhi for Students and Children in English, 10 Lines on Satya Nadella for Students and Children in English, 10 Lines on Manu Bhaker for Students and Children in English, Sardar Vallabhbhai Patel Essay for Students and Children in English, 10 Lines on Narendra Modi for Students and Children in English, 10 Lines on Pandit Jawaharlal Nehru for Students and Children in English, 10 Lines on M Fathima Beevi for Students and Children in English, 10 Lines on Chandrashekhar Azad for Students and Children in English, 10 Lines on Kiran Bedi for Students and Children in English, 10 Lines on Bhagat Singh for Students and Children in English, 10 Lines on Khudiram Bose for Students and Children in English, A conductor in the form of a wire, rod or plate which.
that can be asked in the final exam. Thursday, 10 September 2020. Analysing the Electrolysis of Aqueous Solutions. Copper sulphate solution is electrolyzed using copper electrodes.
Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? 2 (l) Pb (s) + Br 2 (g) Equations. 3. Would spinning bush planes' tundra tires in flight be useful? Once molten, two carbon electrodes, which are attached to a 12V power supply, are placed in the clear and colourless molten liquid. WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced
Fill in the blank :The _______ the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode. Anode : OH- - e- OH 4OH- 2H2O + H2 4.