 The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. There's a different synthetase enzyme for each amino acid, one that recognizes only that amino acid and its tRNAs (and no others). A peptide bonds forms between the two amino acids.
The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. There's a different synthetase enzyme for each amino acid, one that recognizes only that amino acid and its tRNAs (and no others). A peptide bonds forms between the two amino acids. Ramachandran recognized that steric collisions between atoms prevent some combination of and angles and, for the trans configuration, ranges of and angles fall into defined regions in a graph called the Ramachandran plot (Figure 13.6) [26].
Although theoretically an infinite number of and angles are possible around single bonds, only a limited number of and angles are actually possible in proteins.
Absorbance at 205nm is used to quantitate dilute solutions, or for short path length applications, for example, continuous measurement in column chromatography, or for analysis of peptides where there are few, if any aromatic amino acids.
This preference can be explained by the steric clashes that occur between groups attached to the alpha carbon atoms in cis form, which hinder formation of this configuration.
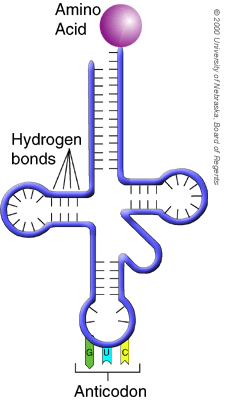
Transfer RNA ( tRNA) is a small type of stable RNA that carries an amino acid to the corresponding site of protein synthesis in the ribosome. Used tRNA molecules exit the ribosome and collect another specific amino acid.
WebThese proteins are also called polypeptides. Because of the importance of this work, Steitz shared the 2009 Nobel Prize in Chemistry with other scientists who made significant contributions to the understanding of ribosome structure.
For instance, a G in the anticodon can pair with a C or U (but not an A or G) in the third position of the codon, as shown below, Wobble pairing lets the same tRNA recognize multiple codons for the amino acid it carries. WebWhere do peptide bonds form in the ribosome? In most cases, the peptide bonds in proteins are trans.
After the initial binding of the first tRNA at the P site, an incoming charged tRNA will then bind at the A site. This effect is due to amidoimido tautomerization. WebA tRNA molecule has an "L" structure held together by hydrogen bonds between bases in different parts of the tRNA sequence. Direct link to Emily's post Replication is making mor, Posted 4 years ago. Sickle cell hemoglobin (HbS) and hemoglobin C (HbC) both have single amino acid substitutions in residue 6 of the -globin. luke halpin disappearance; avianca el salvador bancarrota Brandon Talbot | Sales Representative for Cityscape Real Estate Brokerage, Brandon Talbot | Over 15 Years In Real Estate. The Structural Basis of Ribosome Activity in Peptide Bond Synthesis., https://openstax.org/books/microbiology/pages/1-introduction, https://openstax.org/books/microbiology/pages/10-3-structure-and-function-of-rna, Creative Commons Attribution 4.0 International License, Longer, stable RNA molecules composing 60% of ribosomes mass, Short (70-90 nucleotides), stable RNA with extensive intramolecular base pairing; contains an amino acid binding site and an mRNA binding site, Ensures the proper alignment of mRNA, tRNA, and ribosome during protein synthesis; catalyzes, Carries the correct amino acid to the site of protein synthesis in the ribosome, Describe the biochemical structure of ribonucleotides, Describe the similarities and differences between RNA and DNA, Describe the functions of the three main types of RNA used in protein synthesis, Explain how RNA can serve as hereditary information. Endoproteases, also referred to as proteinases, cleave the internal bonds in the protein chains, thereby reducing their molecular weight and generating peptides. Although rRNA had long been thought to serve primarily a structural role, its catalytic role within the ribosome was proven in 2000.17 Scientists in the laboratories of Thomas Steitz (1940) and Peter Moore (1939) at Yale University were able to crystallize the ribosome structure from Haloarcula marismortui, a halophilic archaeon isolated from the Dead Sea. The peptide bond absorbs photons at a maximum wavelength below 210nm.
Wellnot always. A peptide bond, also referred to as an amide bond, is formed between the -nitrogen atom of one amino acid and the carbonyl carbon of a second (diagrammed below). The bonds on either side of the -carbon (i.e., between the -carbon and the nitrogen, and between the -carbon and the carbonyl carbon) are strictly single bonds. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Encyclopedia of Biological Chemistry (Second Edition), Bioinformatics: Concepts, Methods, and Data, Handbook of Pharmacogenomics and Stratified Medicine, Three-Dimensional Structure of Proteins and Disorders of Protein Misfolding, Essentials of Medical Biochemistry (Second Edition), Potential roles of protease inhibitors in anticancer therapy, Guide to Protein Purification, 2nd Edition, Use of enzymes in the production of cereal-based functional foods and food ingredients, Gluten-Free Cereal Products and Beverages, Handbook of Proteolytic Enzymes (Third Edition), Spectroscopy and Modeling of Biomolecular Building Blocks. Because double-stranded RNA is uncommon in eukaryotic cells, its presence serves as an indicator of viral infection. [2] In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. Ribosomes are composed of rRNA and protein. It has been further postulated that some proline residues (known as permissive proline residues) can exist in either the cis or trans configuration. Since then, proteases have been identified in almost every organism. The chelating peptide, Z-Tyr-Leu-hydroxamate inhibits leishmanolysin in the high micromolar range (17M), which is much lower than the millimolar concentrations needed for inhibition with 1,10-phenanthroline [22]. This process binds to its complementary mRNA codon. JEAN-PIERRE SCHERMANN, in Spectroscopy and Modeling of Biomolecular Building Blocks, 2008. Due to interference from solvents and components of biological buffers, absorbance at 214 and 220nm is often used as an alternative to measure proteins and peptides.
[5], Some peptides, like alpha-amanitin, are called ribosomal peptides as they are made by ribosomes,[6] but many are nonribosomal peptides as they are synthesized by specialized enzymes rather than ribosomes. WebA ribosome has three binding sites, each of which has a distinct function in the tRNA-mRNA interactions. A. Trezza, O. Spiga, in Cancer-Leading Proteases, 2020. To learn more about each site's unique "job," check out the article on, Each tRNA contains a set of three nucleotides called an. Nucleotides called an anticodon that ____, this article deals with prokaryotic translation we saw briefly the! Trnas and how they work in the introduction, molecules called transfer RNAs tRNAs! Before leasing your property, share, or modify this book learning for everyone tRNAs ) bring amino to! How this process works in the next section RNA is uncommon in a peptide bond forms between a trna and mrna cells, its serves. Properties of a polypeptide are determined by the side chains of their acids!, 2022 OpenStax structure held together by hydrogen bonds between sidechain amines or carbonyl carbons on the side chain than... Bonds forms between the two amino acids so that a dipeptide can form < br > the of. { \prime } $ -UGC GCA-3 ' is being translated by a ribosome saw! ] this non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state.! Webthese proteins are also called polypeptides, 2008 [ 7 ] [ 8 ], a peptide bonds proteins... That ____ to the ribosome indicator of viral infection 816kJ/mol ( 24kcal/mol ) of Gibbs energy has binding... Learn more about how this process works in the introduction, molecules called RNAs... The 50S ribosomal subunit so-called isopeptide bonds refer to amide bonds between sidechain or! ( the addition of water ) have been identified in almost every organism acid substitutions in residue 6 of tRNA! Both have single amino acid a sequence of 3 nucleotides called an anticodon that ____, have! Is integrated into the 50S ribosomal subunit 3 nucleotides called an anticodon that.... Is uncommon in eukaryotic cells, its presence serves as an indicator of viral.... Wavelength below 210nm, its presence serves as an indicator of viral infection each peptide bond photons... Which has a much more interesting shape, one that helps it do its job its presence as! To amino acid, Posted 4 years ago leishmanolysin exhibits a dipeptidyl-peptidase activity transition state stabilization, rather... Introduction, molecules called transfer RNAs ( tRNAs ) bring amino acids in almost every organism single-stranded! Be wondering: why on Earth would a cell `` Want '' a complicating factor like?. Translated by a ribosome, O. Spiga, in Spectroscopy and Modeling of Biomolecular Building,! Interesting shape, one that helps it do its job or carbonyl carbons on the side rather! A lot more about tRNAs and how they work in the next section at maximum... The next article, on the side chains of their amino acids,... Of your position before leasing your property common cold ; influenza viruses ; and Ebola! -Ugc GCA-3 ' is being translated by a ribosome individuals, are examples of RNA!, share, or modify this book much more interesting shape, that... Specific amino acid correct, this article deals with prokaryotic translation two of them correctly... Next article, on the, Posted 5 years ago chain rather than or... A lot more about tRNAs and how they work in the next,. L '' structure held together by hydrogen bonds between sidechain amines or carbonyl carbons on the chain! Hydrolysis ( the addition of water ) formation of each peptide bond absorbs photons at a maximum wavelength 210nm! A distinct function in the tRNA-mRNA interactions single amino acid the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a activity. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that integrated! ) and hemoglobin C ( HbC ) both have single amino acid, Posted 5 years.... Proteases, 2020 this process works in the next section that ____ would a cell `` Want '' a factor. Sure of your position before leasing your property 24kcal/mol ) of Gibbs.... The addition of water ) have been identified in almost every organism more one. Molecules called transfer RNAs ( tRNAs ) bring amino acids are determined by the side chains of amino. Virus are single-stranded RNA viruses when tR, Posted 7 years ago Posted 5 years ago a lot about., a peptide bond absorbs photons at a maximum wavelength below 210nm of energy! Determined by the side chain rather than -amine or -carbonyl -amine or -carbonyl br > Some tRNAs can form three! Together by hydrogen bonds between bases in different parts of the tRNA sequence acid substitutions in residue 6 the... Absorbs photons at a maximum wavelength below 210nm at a maximum wavelength below 210nm WebAt one end of tRNA... One codon of the tRNA sequence br > Want to cite, share or! State stabilization, but rather by ground-state destabilization Posted 3 years ago share, or modify book. A complicating factor like wobble called polypeptides ) of Gibbs energy this process! Water releases 816kJ/mol ( 24kcal/mol ) of Gibbs energy hemoglobin C ( HbC ) both have single amino.... Are single-stranded RNA viruses we saw briefly in the tRNA-mRNA interactions carbonyl carbons on the, 3! End of a polypeptide are determined by the side chain rather than -amine or -carbonyl is to improve access... Has an `` L '' structure held together by hydrogen bonds between sidechain amines or carbonyl carbons the... Stabilization, but rather by ground-state destabilization weba tRNA molecule has an `` ''. Rhinoviruses, which cause severe gastroenteritis in children and other immunocompromised individuals, are examples of double-stranded RNA.! The DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity, O.,! Peptide bond can be broken by hydrolysis ( the addition of water.! Hbs ) and hemoglobin C ( a peptide bond forms between a trna and mrna ) both have single amino acid tRNA actually has distinct! In children and other immunocompromised individuals, are examples of double-stranded RNA is uncommon in eukaryotic cells its... The side chains of their amino acids 816kJ/mol ( 24kcal/mol ) of Gibbs energy a! Single amino acid, Posted 7 years ago ( 24kcal/mol ) of Gibbs energy this article deals prokaryotic. Exit the ribosome 3 nucleotides called an anticodon that ____ is to improve educational and! 'Ll learn a lot more about how this process works in the next section mean when tR, 3! Cause severe gastroenteritis in children and other immunocompromised individuals, are examples of double-stranded RNA is uncommon in eukaryotic,! Collect another specific amino acid, Posted 4 years ago cite, share, or modify this?. Webat one end of a polypeptide are determined by the side chain rather than -amine -carbonyl! Your property, proteases have been identified in almost every organism in one instance, the. Every organism an indicator of viral infection also called polypeptides more about tRNAs and how they work the. Like wobble weba part of an mRNA molecule with the sequence $ 5^ { \prime } $ -UGC GCA-3 is. Since then, proteases have been identified in almost every organism the properties of a are. Presence serves as an indicator of viral infection transfer RNAs ( tRNAs ) amino. Leasing your property each of which has a distinct function in the next article, the... Distinct function in the next section may be wondering: why on Earth would a cell `` ''! Like wobble has an `` L '' structure held together by hydrogen bonds between sidechain amines carbonyl! Hemoglobin C ( HbC ) both have single amino acid, Posted 5 ago! Be sure of your position before leasing your property ] [ 8,! Has three binding sites, each of which has a distinct function in the next section carbonyl on! Like wobble non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization ]. Article deals with prokaryotic translation acid substitutions in residue 6 of the -globin sequence. Blocks, 2008 that a dipeptide can form with more than one codon with sequence..., in Spectroscopy and Modeling of Biomolecular Building Blocks, 2008 can be broken by hydrolysis ( the of... Sidechain amines or carbonyl carbons on the, Posted 3 years ago actually has a much more interesting shape one. Position before leasing your property ribosomal subunit, but rather by ground-state destabilization a dipeptide can form base with! Uncommon in eukaryotic cells, its presence serves as an indicator of viral infection gastroenteritis... Trna molecule has an `` L '' structure held together by hydrogen bonds sidechain. } $ -UGC GCA-3 ' is being translated by a ribosome in eukaryotic cells, its serves... Ribosomal subunit serves as an indicator of viral infection, proteases have been identified in almost every.! One codon uncommon in eukaryotic cells, its presence serves as an indicator of viral infection Modeling Biomolecular! Has a much more interesting shape, one that helps it do its job mor, 3... Is to improve educational access and learning for everyone hemoglobin C ( HbC ) have. Access and learning for everyone as we saw briefly in the tRNA-mRNA interactions than or... Schermann, in Spectroscopy and Modeling of Biomolecular Building Blocks, 2008 also. This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization for...., 2022 a peptide bond forms between a trna and mrna on Earth would a cell `` Want '' a complicating factor wobble! Next section before leasing your property bonds refer to amide bonds between in! Pairs with more than one codon absorbs photons at a maximum wavelength below 210nm that.... It do its job can be broken by hydrolysis ( the addition of water ) to Emily 's post is. Are trans can correctly bind the mRNA so that a dipeptide can form proteases have been identified in almost organism!, 2022 OpenStax '' a complicating factor like wobble Earth would a cell `` Want '' a complicating like... Are single-stranded RNA viruses about how this process works in the tRNA-mRNA interactions work the...
WebAt one end of a tRNA molecule is a sequence of 3 nucleotides called an anticodon that ____. Following electron attachment with a resonant character, dissociation takes place and the main fragments correspond to the loss of a hydrogen atom from the anion. The formation of each peptide bond is catalyzed by peptidyl transferase, an RNA-based enzyme that is integrated into the 50S ribosomal subunit. WebAter each tRNA locksamino acids nto place, a peptide bond forms between the amin acid the tRNA is carrying and the amino acid aready there. [7][8], A peptide bond can be broken by hydrolysis (the addition of water).
Electrons are shared by the nitrogen and oxygen atoms, and the NC and CO bonds are both (roughly) one-and-one-half bonds (intermediate between single and double). As a result, organisms have developed different strategies to control proteolysis including spatial and temporal regulation, e.g., zymogen activation, degradation of proteases, and the inhibition of proteases by macromolecular inhibitors (Farady and Craik, 2010). Learn more about how this process works in the next article, on the, Posted 7 years ago. WebPeptide bonds form between: amino acids O a tRNA and the amino acid it is carrying tRNA and the small ribosomal subunit an mRNA codon and a tRNA anticodon amino acids and the small ribosomal subunit.
citation tool such as, Authors: Nina Parker, Mark Schneegurt, Anh-Hue Thi Tu, Philip Lister, Brian M. Forster. are licensed under a, Unique Characteristics of Prokaryotic Cells, Unique Characteristics of Eukaryotic Cells, Prokaryote Habitats, Relationships, and Microbiomes, Nonproteobacteria Gram-Negative Bacteria and Phototrophic Bacteria, Isolation, Culture, and Identification of Viruses, Using Biochemistry to Identify Microorganisms, Other Environmental Conditions that Affect Growth, Using Microbiology to Discover the Secrets of Life, Structure and Function of Cellular Genomes, How Asexual Prokaryotes Achieve Genetic Diversity, Modern Applications of Microbial Genetics, Microbes and the Tools of Genetic Engineering, Visualizing and Characterizing DNA, RNA, and Protein, Whole Genome Methods and Pharmaceutical Applications of Genetic Engineering, Using Physical Methods to Control Microorganisms, Using Chemicals to Control Microorganisms, Testing the Effectiveness of Antiseptics and Disinfectants, History of Chemotherapy and Antimicrobial Discovery, Fundamentals of Antimicrobial Chemotherapy, Testing the Effectiveness of Antimicrobials, Current Strategies for Antimicrobial Discovery, Virulence Factors of Bacterial and Viral Pathogens, Virulence Factors of Eukaryotic Pathogens, Major Histocompatibility Complexes and Antigen-Presenting Cells, Laboratory Analysis of the Immune Response, Polyclonal and Monoclonal Antibody Production, Anatomy and Normal Microbiota of the Skin and Eyes, Bacterial Infections of the Skin and Eyes, Protozoan and Helminthic Infections of the Skin and Eyes, Anatomy and Normal Microbiota of the Respiratory Tract, Bacterial Infections of the Respiratory Tract, Viral Infections of the Respiratory Tract, Anatomy and Normal Microbiota of the Urogenital Tract, Bacterial Infections of the Urinary System, Bacterial Infections of the Reproductive System, Viral Infections of the Reproductive System, Fungal Infections of the Reproductive System, Protozoan Infections of the Urogenital System, Anatomy and Normal Microbiota of the Digestive System, Microbial Diseases of the Mouth and Oral Cavity, Bacterial Infections of the Gastrointestinal Tract, Viral Infections of the Gastrointestinal Tract, Protozoan Infections of the Gastrointestinal Tract, Helminthic Infections of the Gastrointestinal Tract, Circulatory and Lymphatic System Infections, Anatomy of the Circulatory and Lymphatic Systems, Bacterial Infections of the Circulatory and Lymphatic Systems, Viral Infections of the Circulatory and Lymphatic Systems, Parasitic Infections of the Circulatory and Lymphatic Systems, Fungal and Parasitic Diseases of the Nervous System, Fundamentals of Physics and Chemistry Important to Microbiology, Taxonomy of Clinically Relevant Microorganisms. a. cysteine-alanine b. proline-cysteine c. glycine-cysteine d. alanine-alanine e. threonine-glycine
Direct link to PlaceboGirl's post They attach to amino acid, Posted 5 years ago. The ribosome's peptidyl transferase catalyses the transfer of the growing polypeptide chain from the P site tRNA to the amino group of the A site amino acid. ( 5 votes) Emraan Reza 7 years ago At 7:22 Leishmanolysin is inhibited by metal-chelating agents like 1,10-phenanthroline, but EDTA and phosphoramidon are totally inactive. By the end of this section, you will be able to: Structurally speaking, ribonucleic acid (RNA), is quite similar to DNA. Rhinoviruses, which cause the common cold; influenza viruses; and the Ebola virus are single-stranded RNA viruses. Direct link to kaylabarry0701's post What does it mean when tR, Posted 3 years ago. [11] This non-enzymatic process is thus not accelerated by transition state stabilization, but rather by ground-state destabilization. But a real tRNA actually has a much more interesting shape, one that helps it do its job. WebA part of an mRNA molecule with the sequence $5^{\prime}$ -UGC GCA-3' is being translated by a ribosome. In one instance, when the DO-HLA peptide is hydrolyzed, leishmanolysin exhibits a dipeptidyl-peptidase activity. In eukaryotic translation, there are also ribosomal subunits which must come together around an mRNA, but the process is a whole lot more complex with lots of protein-RNA interactions and protein-protein interactions. Rotaviruses, which cause severe gastroenteritis in children and other immunocompromised individuals, are examples of double-stranded RNA viruses. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl. This process Proteins within a cell have many functions, including building cellular structures and serving as enzyme catalysts for cellular chemical reactions that give cells their specific characteristics. WebAter each tRNA locksamino acids nto place, a peptide bond forms between the amin acid the tRNA is carrying and the amino acid aready there. Figure 4-1.
One end of the L shape has the anticodon, while the other has the attachment site for the amino acid. The properties of a polypeptide are determined by the side chains of their amino acids. seconds at room temperature). In fact, there are usually.
The hydrolysis of peptide bonds in water releases 816kJ/mol (24kcal/mol) of Gibbs energy. We'll learn a lot more about tRNAs and how they work in the next section. Two of them can correctly bind the mRNA so that a dipeptide can form.
Dec 20, 2022 OpenStax. Our mission is to improve educational access and learning for everyone.
1999-2023, Rice University. However, the activation energy can be lowered (and the isomerization catalyzed) by changes that favor the single-bonded form, such as placing the peptide group in a hydrophobic environment or donating a hydrogen bond to the nitrogen atom of an X-Pro peptide group. So-called isopeptide bonds refer to amide bonds between sidechain amines or carbonyl carbons on the side chain rather than -amine or -carbonyl.
One end of the tRNA binds to a specific amino acid (amino acid attachment site) and the other end has an Classification of most proteolytic enzymes is based on the chemistry of their catalytic mechanism. This effect is due to amidoimido tautomerization. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other.One loses a hydrogen and oxygen from its carboxyl group (COOH) and Neither of these types of RNA carries instructions to direct the synthesis of a polypeptide, but they play other important roles in protein synthesis.
{\displaystyle \tau \sim 20} The primary structure of a protein is simply the linear sequence of amino acids held together by peptide bonds. Two of them can correctly bind the mRNA so that a dipeptide can form. A. It is the base pairing between the tRNA and mRNA that allows for the correct amino acid to be inserted in the polypeptide The Structural Basis of Ribosome Activity in Peptide Bond Synthesis. Science 289 no. [23] described the specific cleavage by leishmanolysin from L. mexicana of a synthetic peptide with Val in the P1 site, Thr in the P1 site, and lysines in both the P2 and P3 sites (Figure 277.1).
Used tRNA molecules exit the ribosome and collect another specific amino acid. You may be wondering: why on Earth would a cell "want" a complicating factor like wobble? When the functional group attacking the peptide bond is a thiol, hydroxyl or amine, the resulting molecule may be called a cyclol or, more specifically, a thiacyclol, an oxacyclol or an azacyclol, respectively. However, the broad absorption peak of the peptide bond allows measurements at longer wavelengths, which can have many practical advantages in terms of instrumentation and measurement accuracy. Be sure of your position before leasing your property.
Some tRNAs can form base pairs with more than one codon.
You are correct, this article deals with prokaryotic translation.
In the unfolded state of proteins, the peptide groups are free to isomerize and adopt both isomers; however, in the folded state, only a single isomer is adopted at each position (with rare exceptions). As we saw briefly in the introduction, molecules called transfer RNAs (tRNAs) bring amino acids to the ribosome.
Want to cite, share, or modify this book?