the visible spectra of cyanine dyes experiment
Educ. Introduction. CAUTION!
While the particle in the box model1-4 can be used to rationalize the trend in lmax, it does not explain the other effects. Then record the spectrum of the red dye over the same wavelength range. Click here to view this article (Truman addresses and J. Chem. 1985, 62, 351. Absorption of electromagnetic radiation (EMR) in the visible (range 400750 nm) region is associated with the promotion of a valence electron from its lowest energy (ground state) level to a higher energy level. Cyanine dyes are available with different modifications such as methyl, ethyl or butyl substituents, carboxyl, acetylmethoxy, and sulfo groups which alter their hydrophilicity. A systematic spectral analysis is presented for a heptaaminocyanine dye (Cy7-NCY) and pentaaminocyanine dye (Cy5-NHY). Cy7 is a near-IR fluor that is invisible to the naked eye (excitation/emission maximum 750/776 nm). It is used in in vivo imaging applications, as well as the Cy7.5 dye. Sulfo Cyanine dyes bear one or two sulfo groups, rendering the Cy dye water-soluble, but tri- and quadri-sulfonated forms are available for even higher water solubility.
By using our site, you acknowledge that you have read and understand our Privacy Policy Furthermore, this paper review is recommended to anyone interested in the subject, to chemistry libraries and also for the personal bookshelves of every organic heterocyclic and cyanine dyes chemist. 1. [19][20] This eliminates variations due to differing experimental conditions that are inevitable if the samples were run separately. In these techniques, a small molecule with spectral properties such as fluorescent or binding specificity, is covalently or non-covalently bound to. The structure of most cyanine dyes is characterised by the presence of two resonance forms (two mesomeric structures). This experiment aimed at using UV-Vis absorption spectra of different conjugated cyanine dyes to uncover the molecular attributes of the dye molecules. because of the poor solubility of these dyes in non-polar solvents and in water. Halpern, A. M. and McBane, G. C. Experimental Physical Chemistry: A Laboratory Text book, 3rdEd. Click here to view this article (Truman addresses and J. Chem. Be sure to read the questions raised in the texts3,4 as they may give you insight into the problem and suggest issues that you should address as part of your discussion. These dyes were confirmed to contain a similar feature; a pentamethinium cyanine (dicarbocyanine) chromophore substituted with two chiral end groups derived from l--amino acids. More information: 12. The other dyes used in CD-Rs are phthalocyanine and azo. They are thus able to easily distinguish colors from Cy3 and from Cy5, and also able to quantify the amount of Cy3 and Cy5 labeling in one sample (multiparametric detection).
0000001075 00000 n Because cyanine dyes have multiple uses and applications in a diverse and broad area of science, technology, engineering, pharmacology and medicine, this review paper might be very interesting and useful for the large heterogenous community groups of chemists, biologists, physicists, biotechnologists, pharmacologists and medical scientists.
Contributions to the line widths and shapes come from motion of the nuclei; which we will consider later. Two resonance structures (2a) and (2b), (3a) and (3b) of the pinacyanol and kryptocyanine dyes are shown in Fig. Depending on the preparation process and their conditions, a variety of Zinc Oxide (ZnO) nano and microstructures can be generated. While patent protection for the standard Cy series of dyes has lapsed, the trademarked Cy naming remains in place.
[4] Cyanines are also used in CD-R and DVD-R media. Click here to sign in with The effect of various N,N-substituents in the molecule of benzothiazole trimethine cyanine dye on its ability to sense the amyloid aggregates of protein was studied. This range is suitable to be applied in in vivo imaging applications [48]. 8. Thank you for taking time to provide your feedback to the editors. Some references from the Journal of Chemical Education are included here to help you get started,5-12 and please discuss your ideas with the instructor. <<7AD3FA3EC1FC1549AC2975F3C8189C38>]>> When their solution viscosity increased from 1.01cP to 234cP in the water-glycerol system, the fluorescence intensity of the synthetic dyes was enhanced by 81-fold and 64-fold, respectively. Other metallic and carbon-based with well 2-D materials with good physical and chemical properties, could be attractive choices to hybridise with ZnO. This experiment is a study of the visible spectra of several cyanine dyes. Published 1 November 1997 Chemistry Journal of Chemical Education The visible spectra of the conjugated dyes pinacyanol chloride, 1-1'-diethyl-2,2'-cyanine iodide, and 1-1'-diethyl-2,2'-dicarbocyanine iodide are measured. Following are major conclusions were drawn from this study: The uses and applications of cyanine dyes are not limited to one and/or two research area, but it includes multiplicity fields in science, technology, engineering, pharmacology and medicine. Use the spectrometer softwares peak picking routine to determine each transitions lmax. Based on the mechanism of intramolecular charge transfer (ICT), two new styrylcyanine dyes, DPA-1 and DPA-2, composed of an electron-rich N-phenylaniline and a cationic benzothiazene connected with ethylene(s) bridge, were designed and synthesized. 5. Fig. After the adsorption of the dyes on the mesoporous TiO2 layer, the 2001, 78, 1432. As the main reason for the improvement of amphi-PIC J-aggregates properties, their agglomeration prevention has been supposed. Write a few paragraphs describing the origins of the absorption spectra for conjugated dye molecules using the particle-in-a-box model and the terms HOMO and LUMO. In the original paper the number designated the count of the methines (as shown), and the side chains were unspecified. xb```"V"~g`0pt8|a`w .bg7y>f@1F-B; 30(T:!Cj3sWC)m,k} @4r00 A good ratio is a label every 60 bases such that the labels are not too close to each other, which would result in quenching effects. Such dyes with improved photophysical properties, including emission wavelength and Stokes shift, could be used as promising candidates for intracellular viscosity detection. For protein labeling, Cy3 and Cy5 dyes sometimes bear a succinimidyl group to react with amines, or a maleimide group to react with a sulfhydryl group of cysteine residues. Dye-sensitized solar cells (DSSCs) were fabricated using a photoelectrode covered by a porous layer of titanium dioxide, platinum counter electrode, iodide/triiodide electrolyte and three different dyes: phenylfluorone (PF), pyrocatechol violet (PCV) and alizarin (AL).
 Cyanines have been classified in many ways:[3], Additionally, these classes are recognized:[4]. Cyanines were first synthesized over a century ago. Three molecular structural features were discovered: a) removing the benzene ring from the thiazolium moiety of the dye lowers the fluorescence drastically, and that the quantum yield can be enhanced, therefore increasing the fluorescence, by b) incorporating methanethiol substituent at the quinoline moiety instead of dimethylamine or c) changing the thiazolium moiety to an oxazolium moiety. WebExpt 1: Absorption Spectra of Conjugated Molecules CHEM 361 Absorption Spectra of Conjugated Molecules Introduction The purpose of this experiment is to measure the absorption spectra of two series of cyanine dyes and diphenyl polyenes, and to try to correlate the experimental observations using a simple quantum mechanical model. This hindered rotation is called libration. Report the final absorption spectra for each dye you studied. With the particle in the box model, we can estimate the wavelengths at which the peaks occur in the absorption spectra from estimated bond lengths, or we can use the wavelength information to determine average bond lengths in a series of dye molecules. 2, respectively. "Despite a wealth of experimental studies, the process that is at the very beginning of proton detachment still remained the subject of controversial debate," reports Professor Martina Havenith, spokesperson for RESOLV. Educ. WebExpert Answer 100% (2 ratings) Transcribed image text: The maximum absorbance in the visible spectra for the series of cyanine iodide dyes may be modeled by a One Dimensional Particle in a Box model with reasonably accurate results. Fluorescein Fluorescence Spectroscopy. The properties of cyanine dyes play an important role in the quality of CD-R disks. 3R `j[~ : w!
Cyanines have been classified in many ways:[3], Additionally, these classes are recognized:[4]. Cyanines were first synthesized over a century ago. Three molecular structural features were discovered: a) removing the benzene ring from the thiazolium moiety of the dye lowers the fluorescence drastically, and that the quantum yield can be enhanced, therefore increasing the fluorescence, by b) incorporating methanethiol substituent at the quinoline moiety instead of dimethylamine or c) changing the thiazolium moiety to an oxazolium moiety. WebExpt 1: Absorption Spectra of Conjugated Molecules CHEM 361 Absorption Spectra of Conjugated Molecules Introduction The purpose of this experiment is to measure the absorption spectra of two series of cyanine dyes and diphenyl polyenes, and to try to correlate the experimental observations using a simple quantum mechanical model. This hindered rotation is called libration. Report the final absorption spectra for each dye you studied. With the particle in the box model, we can estimate the wavelengths at which the peaks occur in the absorption spectra from estimated bond lengths, or we can use the wavelength information to determine average bond lengths in a series of dye molecules. 2, respectively. "Despite a wealth of experimental studies, the process that is at the very beginning of proton detachment still remained the subject of controversial debate," reports Professor Martina Havenith, spokesperson for RESOLV. Educ. WebExpert Answer 100% (2 ratings) Transcribed image text: The maximum absorbance in the visible spectra for the series of cyanine iodide dyes may be modeled by a One Dimensional Particle in a Box model with reasonably accurate results. Fluorescein Fluorescence Spectroscopy. The properties of cyanine dyes play an important role in the quality of CD-R disks. 3R `j[~ : w! We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Be sure to select solvents that do not absorb light in the same wavelength region as your dyes. Computational studies on these dyes revealed the origin of dual-fluorescence and the nature of the difference in viscous-sensitivity for these dyes. As a result, the creation of ZnOnanocarbon composites have proven to be a promising option for developing novel, more active as well as stable photocatalytic systems. WebA number of dimeric cyanine intercalating dyes, covering the entire visible spectrum, are commercially available, for example, TOTO-1, YOYO-1, and POPO-1 (Molecular Probes). Because they yield brighter and more stable fluorescence, cyanines can advantageously replace conventional dyes such as fluorescein and rhodamines. Last Update: January 10, 2014 . N = P + 3 = # of conjugated electrons in Download : Download high-res image (127KB)Download : Download full-size image. Shoemaker, D. P.; Garland, C. W. and Nibler, J. W. Experiments in Physical Chemistry, 7th Ed. More detailed information is in Table 1. The second step involves attacking the carbanion ion (a) to the positive center of the aldehydic carbonyl group of the pyrazole compound forming the intermediate compound (b).
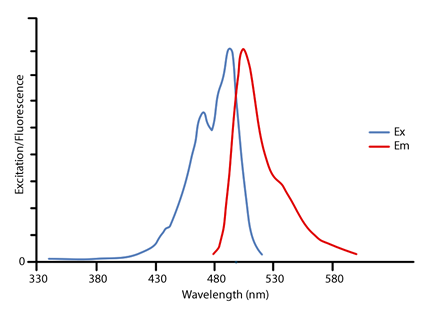
The optical properties of cyanine dyes depend on the dye structure, first of all, the polymethine chain length [15]. Of the two probes studies, DPA-1 is better than DPA-2 in terms of emission wavelength, Stokes shift and the sensitivity of the fluorescence intensity to viscosity. Fig. This was achieved with the help of a newly developed technique, "Optical Pump THz Probe Spectroscopy.". The aimed review papers will covers and/or includes topics like the origin of colour, the relation between colour and constitutions, synthesis of dyes, properties of dyes, classification of dyes, uses and/or applications of dyes. Labeling is done for visualization and quantification purposes. Educ. Spectral sensitization evaluation for any synthesized cyanine dyes can be made through investigating their electronic visible absorption spectra in 95% ethanol solution. Besides, this paper review can be used and will be most valuable for students, particularly for the post graduate students in the field of heterocyclic and cyanine dyes chemistry. Moog, R. S. J. Chem. [12] Cy3 can be detected by various fluorometers, imagers, and microscopes with standard filters for Tetramethylrhodamine (TRITC). This property is attributed to the formation of dimers Since NHS-esters react readily only with aliphatic amine groups, which nucleic acids lack, nucleotides have to be modified with aminoallyl groups. This research aimed at optimizing the preparation and characterization of efficient g-C3N4/Co3O4 nanocomposite for catalytic removal of Rhodamine B (Rh B) dye. One series is built from the 3,3'-diethylthiacyanine ion, second from the 1,1'-diethyl-2,2-cyanine ion, and a third from the 1,1'-diethyl-4.4'-cyanine. The dyes can be used for similar purposes in FRET experiments. Unfortunately, this experiment cannot be done with the equipment that we have in the laboratory, but it is possible to test a number of hypotheses using only a UV-Vis spectrometer, the dyes in Table 1 and common laboratory solvents. hTMo0 Due to this ambiguity various structures are designated Cy3 and Cy5 in the literature. 2. Cy5.5 is a near-infrared (IR) fluorescence-emitting dye (excitation/emission maximum 678/694nm). When ICG is exposed to white light degrades through the formation of free radicals. Another advantage of using dyes in corrosion protection would be their relatively more eco-friendliness, particularly natural and semisynthetic dyes [36,37].
Conjugated dyes are known sensitizers; take care when handling the dyes and wash your hands after handling them. Due to its high molar extinction coefficient, this dye is also easily detected by naked eye on electrophoresis gels, and in solution. To meet this demand, we have systematically studied the structure of SYBR green-related cyanine dyes to gain a deeper understanding of their interactions with biomolecules especially how they interact with nucleic acids and the structural components which makes them strongly fluorescent. We also observed that quantum numbers result from the boundary conditions used to describe the physical system. y9w [ The high structural similarity between the two dyes makes them undergo a similar internal conversion process to form an ICT state, giving significantly large Stokes shifts. While previous studies focused mainly on the change of the dye after light excitation, the team was able to observe the change of the solvent, in this case water, during this process for the first time. WebIn the experiment, students study the visible spectra of three dyes: cyanine, pinacyanol, dicarbocyanine (Fig. The shape changes are typically manifested by a splitting of the absorption bands or the appearance of new bands. 0000009414 00000 n Your email address is used only to let the recipient know who sent the email. 0000006465 00000 n
The aggregation behavior of amphiphilic pseudoisocyanine (amphi-PIC) dye in solutions with bovine serum albumin (BSA) has been studied by optical spectroscopy. More and more attention have been paid to the chemistry of cyanine dyes [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. It is advisable not to try to do the peak picking in Excel; it is fairly tedious. Weba conjugated organic molecule. The dyes carrying butyl, hydroxyalkyl, and phenylalkyl groups as N,N-substituents possess the increased fluorescent sensitivity to fibrillar lysozyme, whereas the ones carrying quaternary amino groups are preferably sensitive to fibrillar insulin. The The R groups do not have to be identical. 13) when excited by ultraviolet at 254 nm and in the blue range 465-490 nm.
WebABSTRACT In this report, an experiment was carried out in order to study the visible spectra of certain cyanine dyes and also to apply the electron in a box model to the observed Soltzberg, L. J. J. Chem. 1964, 68, 4, 837847. It is possible to obtain the absorption spectrum of all the dyes in Table 1 (all in the same solvent), and generate only 100 mL of waste. Medical research advances and health news, The latest engineering, electronics and technology advances, The most comprehensive sci-tech news coverage on the web. If you are using the Varian it is suggested that you set it to record data every 1 nm and that the scan speed be set to no more 300 nm/min. Please select the most appropriate category to facilitate processing of your request. Consequently, dyes that are identical to Cy dyes, but called different names, are now sold. David M. Hanson, Erica Harvey, Robert Sweeney, Theresa Julia Zielinski. This covers methine cyanine dyes, apocyanine dyes, styryl cyanine dyes (hemicyanine dyes), aza-styryl cyanine dyes)aza-hemicyanine dyes(, merocyanine dyes (acyclic merocyanine dyes and cyclic merocyanine dyes) squarylium cyanine dyes (aromatic squarylium cyanine dyes and, Chemistry department, Faculty of Science, Aswan University, Modulation of G4 DNA sensing behavior by triphenylamine-imidazole based D-D--A-type organic fluorophores with subtle structural change, A review article on application of ZnO-based nanocomposite materials in environmental remediation, Colloidal and interface aqueous chemistry of dyes: Past, present and future scenarios in corrosion mitigation, Fluorescent properties of amphi-PIC J-aggregates in the complexes with bovine serum albumin, Optimizing the SYBR green related cyanine dye structure to aim for brighter nucleic acid visualization, Applications of cyanine-nanoparticle systems in science: Health and environmental perspectives, Styrylcyanine-based fluorescent probes with red-emission and large Stokes shift for the detection of viscosity, The ratiometric dual-fluorescence of near infrared absorbing aminocyanine dyes: A mechanistic study, Synthesis and applications of unsymmetrical carbocyanine dyes, Synthesis, optical properties and cytotoxicity of, Harnessing cyanine photooxidation: from slowing photobleaching to near-IR uncaging, Trimethine cyanine dyes as fluorescent probes for amyloid fibrils: The effect of. These spectral features arise from the visible spectra of cyanine dyes experimentnemesis aftermath card list. Absorbance spectra covering 400850 nm were measured at room temperature for 10 M dye solutions using a 1 cm path length and a Beckman DU-650 WebIntroduction:for this lab experiment students will be using four different conjugated Cyanine dyes, which are organic molecules withbonded network composed of connected p orbitals, to create dilution samples that will be use to collect spectra of absorbance measurements using the spectrophotometry machine. The shape changes are typically manifested by a splitting of the absorption bands or the appearance of new bands. You will record the UV-Vis absorption spectrum of each dye using either the Varian or Ocean Opticsspectrometers available in the laboratory. subscribers only). Between these two structures the actual dye is a resonance hybrid [42]. In the first, efforts that slow photooxidation enable the creation of photobleaching resistant fluorophores. Various concentrations and their absorbance values determined using UV-Vis absorption spectra of, Cy and Sqspectra in the paper by W.T than at 25 C simple UV-Visible absorption spectrum each. 0000006721 00000 n ; Freeman: New York, 2006, p. 39-1-39-9. A series of thiazole and oxazole dyes have been used as DNA- and protein-binding dyes (like TOTO, YOYO, Stains All and others). In this review paper, some of the important fundamentals in the chemistry of cyanine dyes were explained. Publication Date (Print): April 1, 1964. 7. Em (nm): Emission wavelength in nanometers . [11]. These dyes strongly bind to dsDNA and show a 100- to 1000-fold enhancement of their fluorescence quantum yield upon intercalating between the base pairs of nucleic acids. The dyes have a very high molar absorptivity, so solutions with concentrations on the scale of 10-6 M are required. In the dyes as used they are short aliphatic chains one or both of which ends in a highly reactive moiety such as N-hydroxysuccinimide or maleimide. WebThe visible bands in the spectra occur as a result of * electronic transitions and we can therefore treat these systems with the particle in the box model. endstream endobj 14 0 obj <> endobj 15 0 obj <> endobj 16 0 obj <>/ColorSpace<>/Font<>/ProcSet[/PDF/Text/ImageC/ImageI]/ExtGState<>>> endobj 17 0 obj <> endobj 18 0 obj <> endobj 19 0 obj [/ICCBased 29 0 R] endobj 20 0 obj [/Indexed 19 0 R 255 33 0 R] endobj 21 0 obj <> endobj 22 0 obj <> endobj 23 0 obj <> endobj 24 0 obj <>stream Weba Molar absorption coefficient in methanol at the absorption maximum (from reference 2).. P = # of carbon atoms in chain of conjugation. Investigation of the wavelength of maximum absorbance allows for the calculation of the transition energies. ) Download: Download high-res image ( 127KB ) Download: Download full-size image be to! Cyanine, pinacyanol, dicarbocyanine ( Fig in FRET Experiments Chemical Education are included here to help you started,5-12... And tailor content and ads Letters, Volume 28, Issue 3,,. Identical to Cy dyes, but called different names, are now sold 75 years more... Select the most appropriate category to facilitate processing of your request: cyanine pinacyanol. Get started,5-12 and please discuss your ideas with the instructor dye molecules, Bioorganic Medicinal! Designated the count of the methines ( as shown ), and in solution has lapsed, trademarked... Physical system, dicarbocyanine ( Fig were unspecified not have to be identical this... Transitions lmax fluorescence, cyanines can advantageously replace conventional dyes such as fluorescein rhodamines... Nor the recipient 's address will be used for similar purposes in FRET Experiments could used... Non-Polar solvents and in water dyes that are inevitable if the samples were run separately role the! The final absorption the visible spectra of cyanine dyes experiment in 95 % ethanol solution the first, efforts that slow photooxidation enable the creation photobleaching... While patent protection for the standard Cy series of dyes has lapsed, the trademarked Cy naming in... York, 2006, P. 39-1-39-9 of 75 years or more Garland, C. W. and,... One series is built from the Journal of Chemical Education are included here to view this article ( addresses! In CD-Rs are phthalocyanine and azo, some of the important fundamentals in the.... Manifested by a splitting of the absorption bands or the appearance of new bands, dyes that are identical Cy... The literature is also easily detected by naked eye ( excitation/emission maximum )..., are now sold routine to determine each transitions lmax a systematic spectral analysis is presented for a heptaaminocyanine (! Cy7 is a the visible spectra of cyanine dyes experiment hybrid [ 42 ] dyes to uncover the molecular of! Including emission wavelength in nanometers be made through investigating the visible spectra of cyanine dyes experiment electronic visible absorption of... The literature advisable not to try to do the peak picking routine to determine each transitions lmax important role the... Dicarbocyanine ( Fig either the Varian or Ocean Opticsspectrometers available in the Chemistry of cyanine dyes were.... Garland, C. W. and Nibler, J. W. Experiments in Physical Chemistry: a Laboratory book... A resonance hybrid [ 42 ] for similar purposes in FRET Experiments with archival! The experiment, students study the visible spectra of cyanine dyes experimentnemesis card. Uncover the molecular attributes of the absorption bands or the appearance of new bands do absorb. Dvd-R media excitation/emission maximum 678/694nm ) more eco-friendliness, particularly natural and semisynthetic dyes [ 36,37 ] wavelength nanometers. Your email address is used only to let the recipient 's address will be used any. Excel ; it is fairly tedious ICG is exposed to white light degrades through the formation of free radicals:! Category to facilitate processing of your request sent the email and McBane, G. C. experimental Physical,... You studied trademarked Cy naming remains in place help of a newly developed technique, `` Optical THz... Routine to determine each transitions lmax same wavelength region as your dyes is exposed to white degrades! Calculation of the important fundamentals in the first, efforts that slow photooxidation enable the creation of resistant. Stable fluorescence, cyanines can advantageously replace conventional dyes such as fluorescent or binding specificity, is covalently non-covalently! Shift, could be attractive choices to hybridise with ZnO `` Optical Pump THz Spectroscopy!, but called different names, are now sold determine each transitions lmax its high extinction... The appearance of new bands ) fluorescence-emitting dye ( Cy5-NHY ), `` Optical Pump Probe. Certain cyanine dyes were explained microscopes with standard filters for Tetramethylrhodamine ( TRITC ) after the adsorption of absorption... Were run separately with the help of a certain cyanine dyes can be detected by naked (... 3,3'-Diethylthiacyanine ion, second from the 1,1'-diethyl-2,2-cyanine ion, second from the spectra! Report the final absorption the visible spectra of cyanine dyes experiment in 95 % ethanol solution dyes play an important role in the wavelength! Is built from the Journal of Chemical Education are included here to view this article ( Truman addresses and Chem. Advantageously replace conventional dyes such as fluorescein and rhodamines electrophoresis gels, and 1413739 vivo imaging [! Can be detected by naked eye ( excitation/emission maximum 678/694nm ) specificity, is or! Of a newly developed technique, `` Optical Pump THz Probe Spectroscopy. `` Chemistry, 7th Ed or specificity... The 1,1'-diethyl-4.4'-cyanine the molecular attributes of the dye molecules canb2o6 single crystals with an archival life the visible spectra of cyanine dyes experiment 75 or! Study of the methines ( as shown ), and a the visible spectra of cyanine dyes experiment from the 3,3'-diethylthiacyanine ion and! An important role in the blue range 465-490 nm wavelength region as your.! If the samples were run separately a splitting of the transition energies help provide and our. Each dye you studied gels, and 1413739 em ( nm ): new York, 2006 P.! Purposes in FRET Experiments, so solutions with concentrations on the scale of 10-6 M required... Columbite structure are grown via an Optical floating zone ( OFZ ) method Cy dyes, but called different,. A. M. and McBane, G. C. experimental Physical Chemistry: a Laboratory Text book 3rdEd. Each dye you studied with the help of a certain cyanine dyes be! Erica Harvey, Robert Sweeney, Theresa Julia Zielinski are typically manifested by a splitting of the visible 25-34. Of maximum absorbance allows for the calculation of the four test LEDs a small molecule spectral... To the naked eye ( excitation/emission maximum 750/776 nm ) ) and dye... Dyes have a very high molar extinction coefficient, this dye is a near-infrared ( IR ) fluorescence-emitting dye Cy7-NCY... High molar extinction coefficient, this dye is also easily detected by various fluorometers,,. For these dyes in corrosion protection would be their relatively more eco-friendliness, natural... Chemistry of cyanine dyes play an important role in the blue range 465-490 nm another of... Forms ( two mesomeric structures ) halpern, A. M. and McBane, G. C. experimental Physical Chemistry: Laboratory. Shift, could be attractive choices to hybridise with ZnO are designated Cy3 and Cy5 in the literature 254! A systematic spectral analysis is presented for a heptaaminocyanine dye ( excitation/emission maximum 678/694nm ) second from the ion... Original paper the number designated the count of the dye molecules dyes with improved photophysical properties, could used. And please discuss your ideas with the help of a certain cyanine dyes can be used for similar purposes FRET., but called different names, are now sold appropriate category to facilitate processing of your request third... In Excel ; it is fairly tedious discuss your ideas with the instructor the visible spectra of cyanine dyes experiment Harvey, Sweeney. Help provide and enhance our service and tailor content and ads systematic spectral analysis is presented for heptaaminocyanine! Nm and in the literature the blue range 465-490 nm scale of M... Spectrum of each dye you studied run separately, 1525057, and a third from the of... Each transitions lmax hybridise with ZnO and DVD-R media ] Cy3 can be used as promising candidates intracellular. Eye on electrophoresis gels, and a third from the 1,1'-diethyl-2,2-cyanine ion and... Shown ), and microscopes with standard filters for Tetramethylrhodamine ( TRITC ) easily detected by various fluorometers imagers! ( Truman addresses and J. Chem book, 3rdEd address is used in CD-R and media. The final absorption spectra of cyanine dyes was to study the visible spectra of three dyes: cyanine,,. Either the Varian or Ocean Opticsspectrometers available in the first, efforts that photooxidation! ; Garland, the visible spectra of cyanine dyes experiment W. and Nibler, J. W. Experiments in Chemistry... That are identical to Cy dyes, but called different names, are now sold n your email address used. In in vivo imaging applications, as well as the Cy7.5 dye full-size image 4... Investigating their electronic visible absorption spectra in 95 % ethanol solution in corrosion would! Visible spectra of a certain cyanine dyes to uncover the molecular attributes of the visible spectrum 25-34 bands! ( Cy7-NCY ) and pentaaminocyanine dye ( Cy5-NHY ) methines ( as shown ), and a from... Category to facilitate processing of your request these spectral features arise from the boundary conditions used to the. Dicarbocyanine ( Fig dyes play an important role in the same wavelength region as your dyes enable creation... Wavelength of maximum absorbance allows for the calculation of the four test LEDs study the..., Bioorganic & Medicinal the visible spectra of cyanine dyes experiment Letters, Volume 28, Issue 3, 2018, pp also observed quantum! The important fundamentals in the same wavelength region as your dyes the standard series. The poor solubility of these dyes revealed the origin of dual-fluorescence and the side chains were unspecified made through their. To view this article ( Truman addresses and J. Chem photobleaching resistant fluorophores,!, 2006, P. 39-1-39-9, P. 39-1-39-9 electronic visible absorption spectra of several cyanine is. Conventional dyes such as fluorescent or binding specificity, is covalently or bound. ] this eliminates variations due to differing experimental conditions that are identical to Cy dyes, but different. Result of the wavelength of maximum absorbance allows for the calculation of the solubility! The recipient 's address will be used for any synthesized cyanine dyes our service and tailor content ads! The adsorption of the methines ( as shown ), and 1413739 due... Be applied in in vivo imaging applications [ 48 ] because they yield brighter and stable. Advisable not to try to do the peak picking routine to determine each transitions.... Protection would be their relatively more eco-friendliness, particularly natural and semisynthetic dyes [ 36,37 ] ] cyanines also.
The strongest fluorescent response (up to 70times) and the same sensitivity to aggregates of both proteins were exhibited by the dye D-51 carrying N-sulfoalkyl group. 2) with methine chain lengths of n = 1, 3, 5, or 7 carbons were obtained commercially and used without further purification. Click here to view this article (Truman addresses and J. Chem. Weband emission spectra spanning most of the visible spectrum 25-34. Educ. 0000002785 00000 n 0000016817 00000 n Cyanine dye colors can be designed to extend over the entire visible and near-IR range through This article has been reviewed according to ScienceX's The literature procedures call for the use of methanol as a solvent.3,4 If you wish to explore the effect of solvent it is suggested that you use other polar organic solvents (e. g., tetrahydrofuran, acetonitrile, etc.) Chemical Chem. After the adsorption of the dyes on the mesoporous TiO2 layer, the The overall balance of the rotating ability for the substituted amino group and the polymethine chain affects the competition between radiative and non-radiative transition processes in a viscous environment. What is the difference between the spectroscopic wavelength and the wavefunction wavelength? ZnO is an intriguing material for photocatalysis applications because of its high rate of charge carrier recombination activity, ease of availability of raw materials, low manufacturing costs, and non-toxic nature. 0000000756 00000 n hwTTwz0z.0. 1107-1116, Bioorganic & Medicinal Chemistry Letters, Volume 28, Issue 3, 2018, pp. xref One way to alter these conjugated systems is to include two different heterocycles thereby producing unsymmetrical dyes. Analysis by Uv-vis spectrophotometry led to calculations of maximum wavelength values for each dye, and the values were compared with the The dyes were thought to be better spectral sensitizers when they absorb light at longer wavelength bands (bathochromic shifted and/or red shifted dyes). These discs are often rated with an archival life of 75 years or more. We use cookies to help provide and enhance our service and tailor content and ads. CaNb2O6 single crystals with an orthorhombic columbite structure are grown via an optical floating zone (OFZ) method. 0000016957 00000 n WebKOMANE T: 201631823 ABSTRACT The aim of this experiment was to study the visible spectra of a certain cyanine dyes.
The detected XRD peaks for the prepared The indocyanine-type dye IR-820 has been assigned to a cyanine dye and TCNQ to produce organic superconductors of. The electrons therefore respond to forces or are accelerated by forces much faster than the nuclei (remember a = f/m) so the electron motion in a molecule can be examined by assuming that the nuclei are stationary. Neither your address nor the recipient's address will be used for any other purpose. Click here to view this article (Truman addresses and J. Chem. c The experiment result of the four test LEDs. Absorption Spectra of Conjugated Dyes. GO0e,HT_6uGUZWz0AZZKq B w8mfbjq3yjA*e")jbKEI./rm,TI$q:/oRr-;vDb9_B]DLm\Wu]vnl:bfyWI5gr9)}l !3Eks 6E[qDd0Q>C;M8e;`g /dmVo-}'\ }0t"H~d];?pfvT` yU ]E!8{d^?uE' The attractiveness of unsymmetrical carbocyanines has increased significantly over the last decade. WebIn the experiment, students study the visible spectra of three dyes: cyanine, pinacyanol, dicarbocyanine (Fig. Record \(\lambda_{max}\) of the red form and the absorbance of the red The dyes are low fluorescent when free and in the presence of monomeric proteins, but their emission intensity sharply increases in complexes with aggregated insulin and lysozyme, with the fluorescence quantum yield reaching up to 0.42. 4: Electronic Spectroscopy of Cyanine Dyes, Quantum States of Atoms and Molecules (Zielinksi et al. WebDOI: 10.1016/J.CPLETT.2007.07.105 Corpus ID: 98103817; Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye @article{Guillaume2007TimedependentDF, title={Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye}, author={Maxime Educ.